Favipiravir intermediate 3, 6-dichloro-2-cyanopyrazine synthesis process
A synthetic process, favipiravir technology, is applied in the field of synthetic process of favipiravir intermediate 3,6-dichloro-2-cyanopyrazine, which can solve the problems of reducing production costs, long synthetic route steps, and yield. Low efficiency and other problems, to achieve the effects of reduced energy consumption, low cost, and convenient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
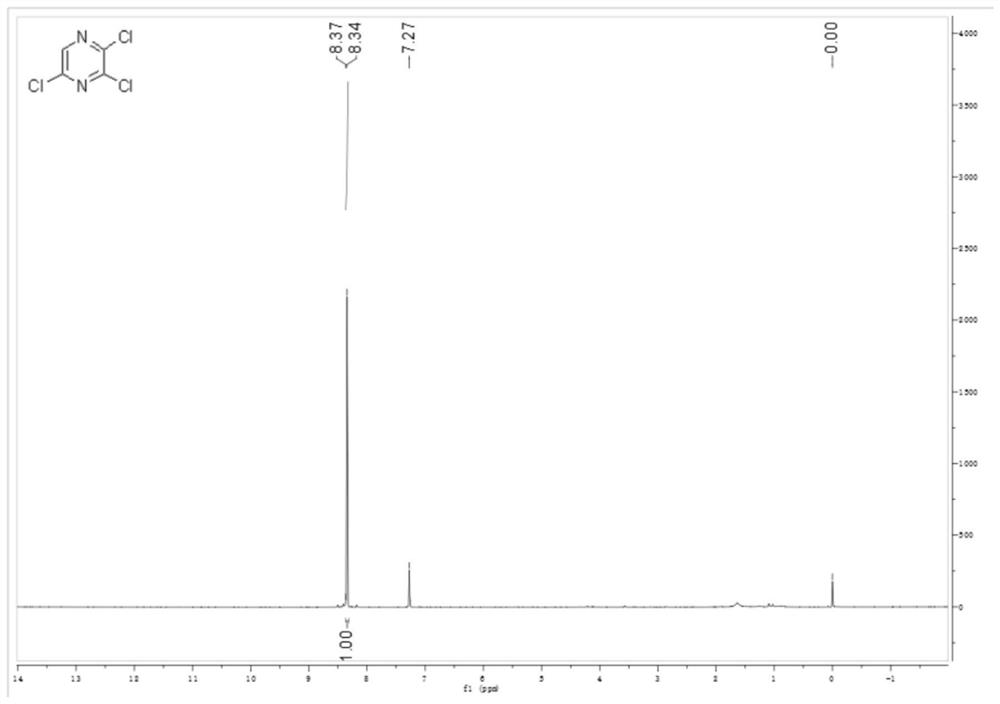
[0044](1) Weigh 100g (0.873mol) of dry 2-chloropyrazine into a 1-liter three-neck flask, replace the nitrogen, add 57.4g (0.786mol) of freshly steamed DMF, control the temperature in an ice bath, and add sulfuryl chloride dropwise at a temperature below 20°C 589g (4.36mol), gas is released, and the addition is completed in about 2 hours; keep at about 20°C for 30 minutes, then raise the temperature to 70°C for 4 hours; HPLC monitors the end of the reaction. Stop the reaction, cool to room temperature, slowly pour the system into 1500g of crushed ice with stirring, extract 300mL of methyl tert-butyl ether each time, extract 3 times in total, dry with anhydrous sodium sulfate, and spin dry to obtain light yellow flaky crystals 125.5 g, purity 97.2%, yield 76.43%. 1 HNMR spectrum see figure 1 .
[0045] (2) Weigh 50g (0.275mol) 2,3,5-trichloropyrazine into a 500ml three-necked flask, add 0.3g (0.55mmol) 1,3-bis(diphenylphosphinopropane) nickel dichloride and 3.3g (8.25mmol) PE...
Embodiment 2
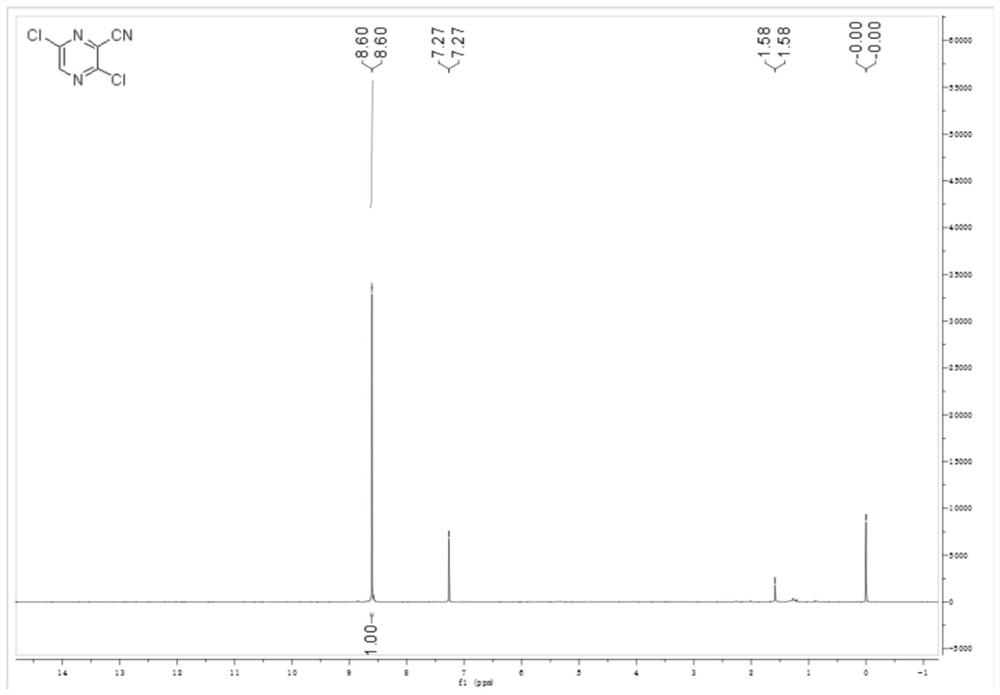
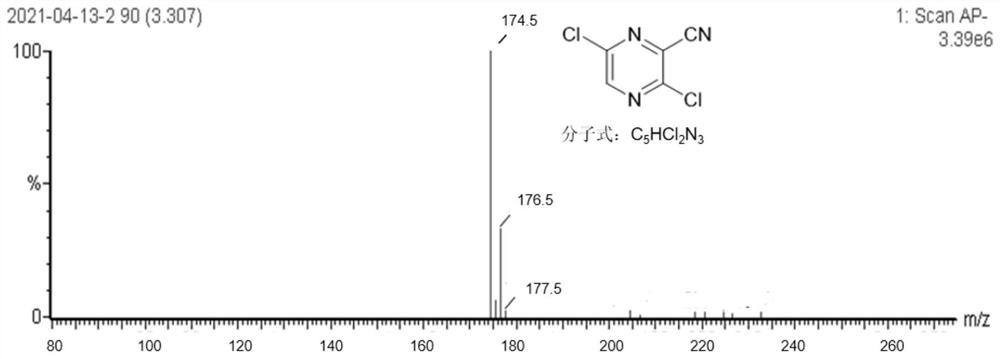
[0047] The other steps are the same as in Example 1, except that 1,3-bis(diphenylphosphinopropane)nickel dichloride is replaced by an equimolar amount of [1,1'-bis(diphenylphosphino)ferrocene]dichloride nickel chloride. The purity of the target product 3,6-dichloro-2-cyanopyrazine was 97.4%, and the total yield was 55.38%.
Embodiment 3
[0049] Other steps are the same as in Example 1, except that PEG400 is replaced by PEG800 in an equimolar amount. The purity of the target product 3,6-dichloro-2-cyanopyrazine was 98.1%, and the total yield was 56.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com