Varicella-herpes zoster vaccine composition as well as preparation method and application thereof
A vaccine composition and herpes zoster technology, applied in the field of vaccines, can solve the problems of difficult quality control in the preparation process, impossibility of artificial synthesis, and high price, so as to promote the phagocytosis of antigen-presenting cells, avoid systemic inflammatory side effects, and promote The effect of the cellular immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment provides a varicella-zoster vaccine composition, the preparation method of which is:
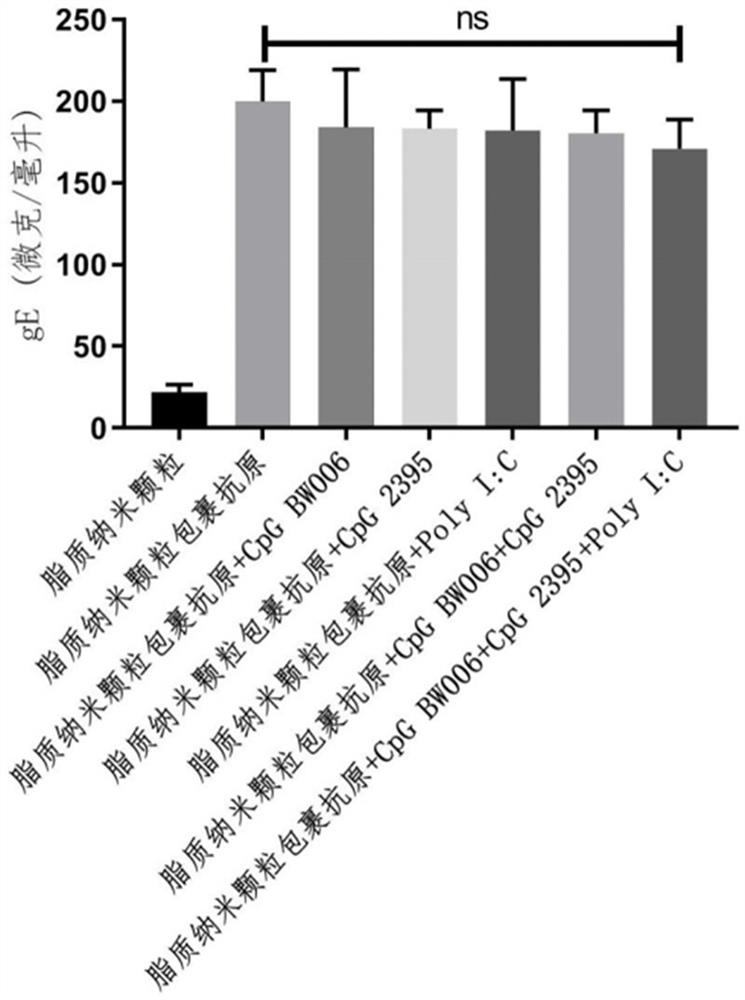
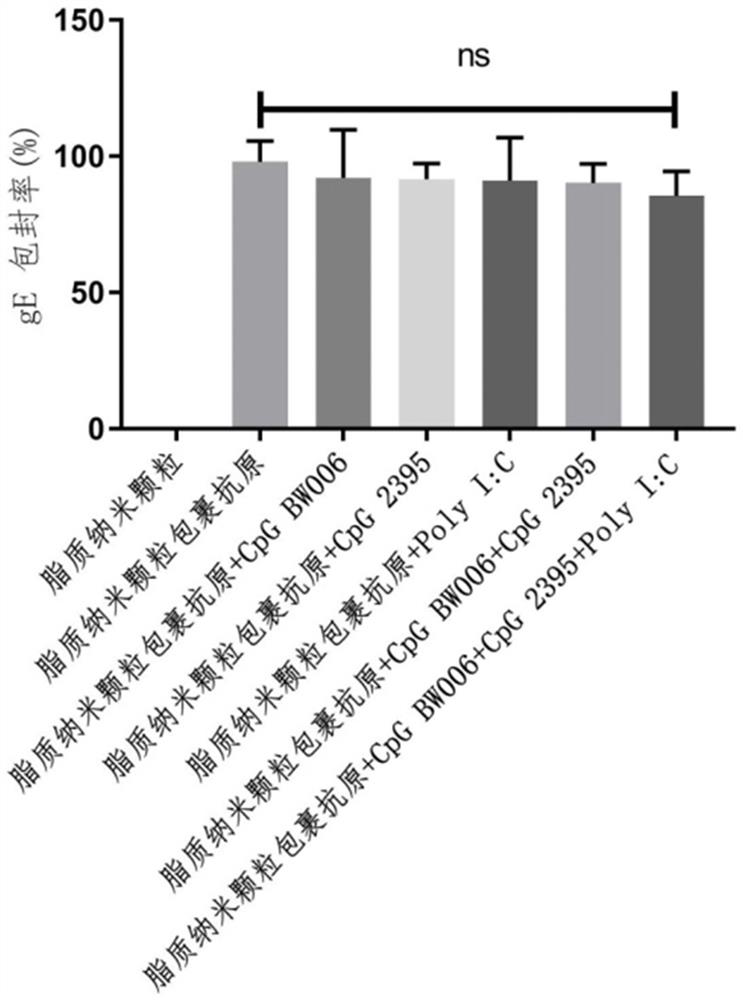
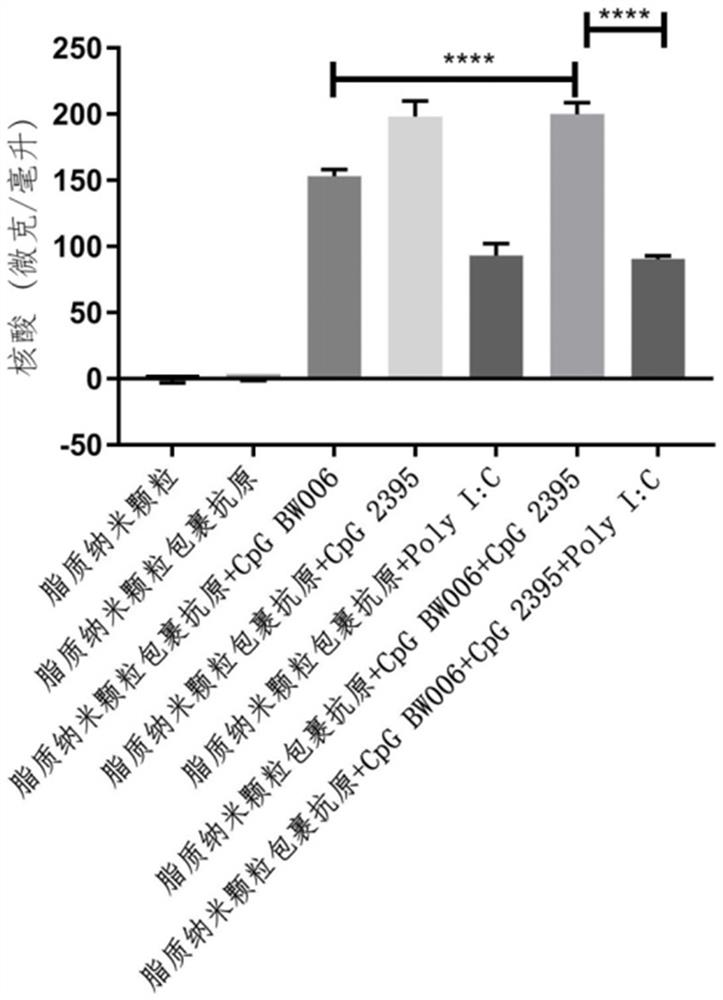
[0041] According to MC3: DSPC: cholesterol: DMG-PEG2000 molar ratio is 50:10:37.5:2.5, the lipid was weighed and dissolved in absolute ethanol to form solution A; 0.3 mg gE, 0.125 mg CpG BW006, 0.125 mg CpG 2395 and 0.125 mg of LMW PolyI:C was dissolved in 100 mM, pH 4.0 citrate buffer to form solution B; using a microfluidic nano-drug manufacturing system (NanoAssemblr Ignite from Precision Nanosystems, Canada) to mix solution A: solution B volume ratio 1:3 , to obtain the varicella-zoster vaccine composition LNP-BW006+2395+PolyI:C-gE.
Embodiment 2
[0043] This embodiment provides a varicella-zoster vaccine composition, the preparation method of which is:
[0044] According to MC3: DSPC: cholesterol: DMG-PEG2000 molar ratio is 50:10:37.5:2.5, weigh the lipid and dissolve it in absolute ethanol. Use the microfluidic nano drug manufacturing system to mix and dissolve 0.3 according to the volume ratio of 1:3. mg gE and 0.4 mg CpG BW006 (synthesized from Shanghai Sangon Bioengineering Co., Ltd.) in 100 mM, pH 4.0 citrate buffer solution to obtain LNP-BW006-gE.
Embodiment 3
[0046] This embodiment provides a varicella-zoster vaccine composition, the preparation method of which is:
[0047] According to MC3: DSPC: cholesterol: DMG-PEG2000 molar ratio is 50:10:37.5:2.5, weigh the lipid and dissolve it in absolute ethanol. Use the microfluidic nano drug manufacturing system to mix and dissolve 0.3 according to the volume ratio of 1:3. mg gE and 0.4 mg CpG 2395 (synthesized from Shanghai Sangon Bioengineering Co., Ltd.) in 100 mM, pH 4.0 citric acid buffer to obtain LNP-2395-gE.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com