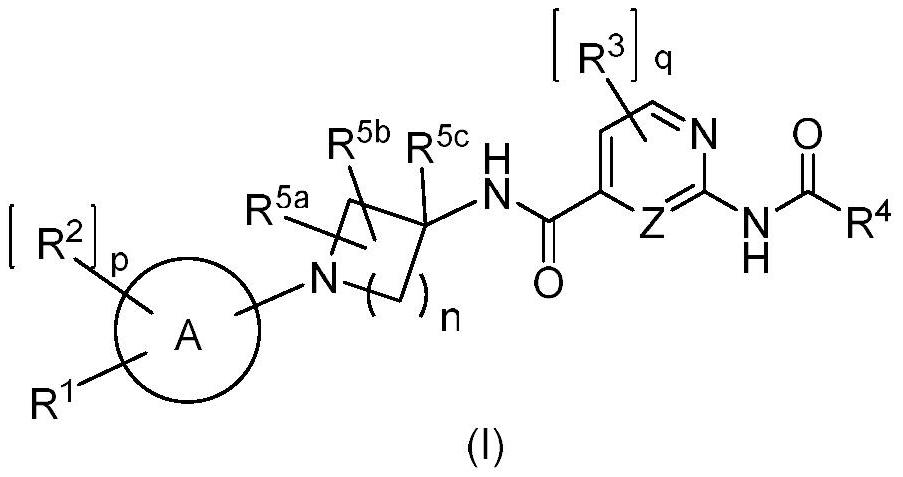
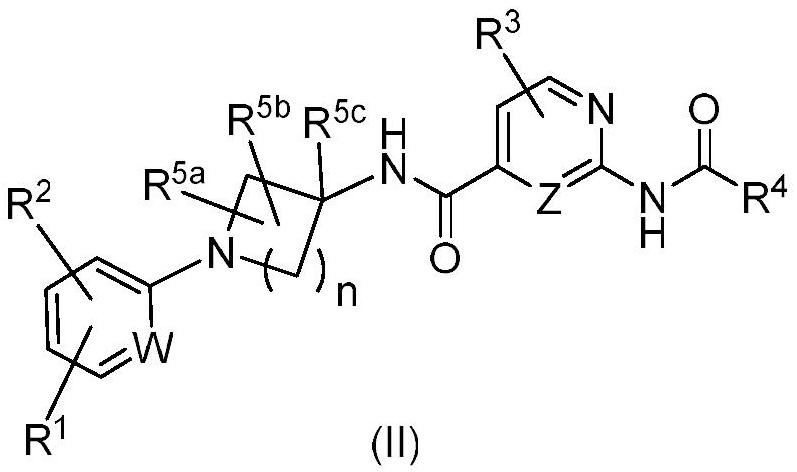
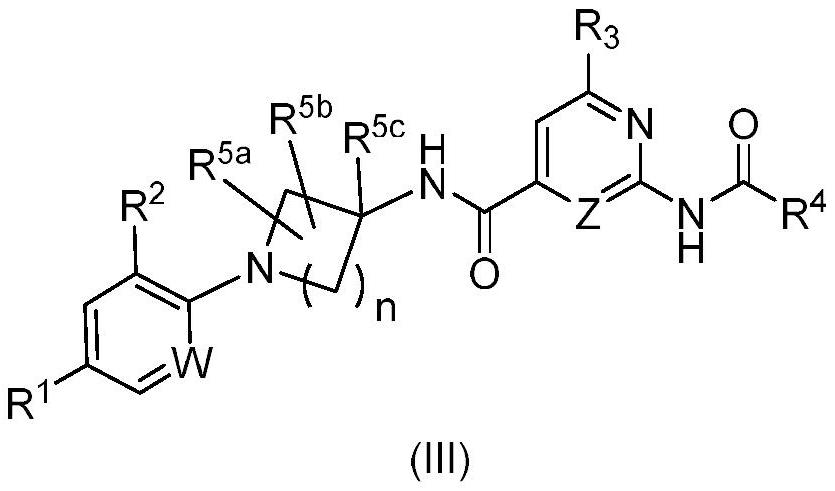
HETEROCYCLIC DERIVATIVES AS Nav1.7 and Nav1.8 BLOCKERS
A compound and cycloalkyl technology, applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as incomplete curative effect, low efficiency, and limitations, and achieve good selectivity, high selectivity, and improved Effect of side effect properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0920] Example 1: (S)-2-acetamido-N-(1-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)pyrrolidin-3-yl)isonicotinamide
[0921] Add (S)-1-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)pyrrolidin-3-amine dihydrochloride (20 mg, 0.059 mmol, amine-1 ), 2-acetamidoisonicotinic acid (11 mg, 0.059 mmol, carboxylic acid-1) and triethylamine (0.041 mL, 0.30 mmol) were mixed in DMF (0.5 mL) and HBTU (27 mg, 0.071 mmol). The mixture was diluted with water (3 mL) and extracted with EtOAc (3 mLx2). The combined organic fractions were dried over sodium sulfate. The organic layer was purified by NH-silica gel column chromatography, eluting with EtOAc, and then by preparative LC-MS to obtain the title compound 6.0 mg (24% yield).
[0922] Regarding the other examples, they were prepared following a method similar to that described in Example 1, using the appropriate amine and carboxylic acid (refer to Table 1). Unless otherwise specified in the synthesis section, the reaction materials are commerc...
Embodiment 2、5、6、7、8、9、10、11、16、20、21、22、23、24、25、26、27、28、37、38、39、41、42、46、47、48、49
[0980] Embodiment 2, 5, 6, 7, 8, 9, 10, 11, 16, 20, 21, 22, 23, 24, 25, 26, 27, 28, 37, 38, 39, 41, 42, 46, 47, 48, 49, 50, 51, 52, 53, 55, 56, 57, 58, 59, 60, 73, 77, 78, 79, 80, 81, 82, 83, 84, 88, 92, 93, 95, 96, 99, 103, 104, 107, 109, 114, 116, 117, 118, 119, 120, 121, 123, 125, 126, 127, 128, 129 and 132.
[0981] FRET analysis
[0982] This screen was used to determine the effect of compounds on human Nav1.8 channel and human Nav1.5 channel using cell imaging technology based on Hamamatsu Photonics' Functional Drug Screening System (FDSS). Changes in membrane potential were monitored by FRET technology using a pair of fluorescent membrane potential dyes, DiSBAC2(3) and CC2-DMPE.
[0983] Cell maintenance:
[0984] HEK293 cells expressing human Nav1.8 channels or human Nav1.5 channels were cultured in T225 flasks under 5% CO 2 Bring to approximately 80% confluence in a humidified incubator. The medium composition of HEK293 cells expressing the channel of human Nav1....
Embodiment 7
[1007] The compound of embodiment 7 is to the IC of Nav1.8 50 The value is 2 μM.
[1008] For all compounds tested, the ratio of activity on Nav1.5 to Nav1.7 or Nav1.8 was more than three-fold. For example, in Example 7, the activity on Nav1.5 and the activity on Nav1.8 were greater than 30 μM and 2 μM, respectively.
[1009] Electrophysiological Analysis
[1010] Whole cell patch clamp recording is used to assess the potency or selectivity of Na (sodium) channel blockers on voltage-gated sodium channels in humans. Use 0.05% Trypsin (Trypsin)-EDTA or Accutase to dissociate Na channel expressing cells, and then inoculate on coverslips for 2-24 hours.
[1011] Manual patch-clamp recordings were performed at room temperature using a voltage-clamp amplifier (Axon Instruments or HEKA electronik). Electrodes were drawn using a P-97 electrode puller (Sutter Instrument). The electrode resistance is 1-3 MΩ when the intracellular solution is full. The current is low-pass filtered ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com