Method for enhancing lentivirus vector production
A technology of lentiviral vectors and lentiviruses, applied in the direction of viruses/bacteriophages, biochemical equipment and methods, viruses, etc., to achieve the effect of improving the production of lentiviral vectors, enhancing the production of lentiviral vectors, and producing them safely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
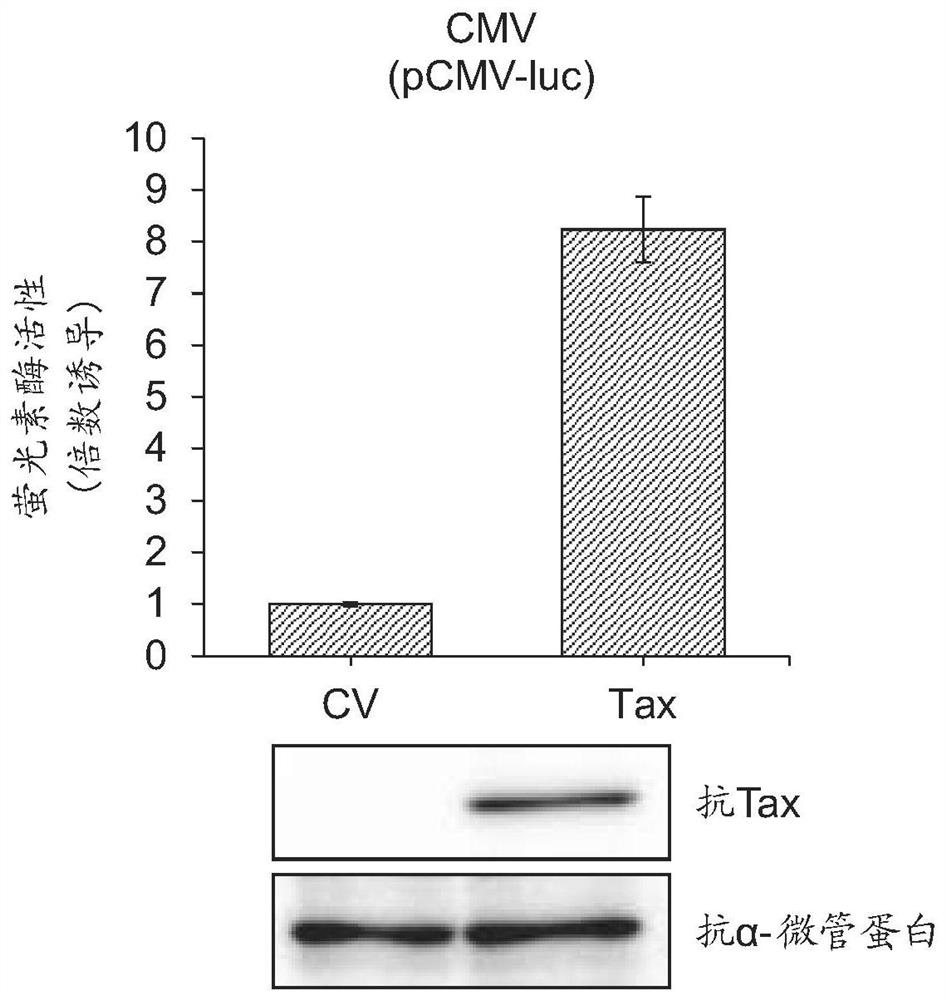
[0109] [Example 1] Enhancement of Lentiviral Vector Production by HTLV-1 Tax
[0110] Materials and methods
[0111] cell
[0112] Cell line HEK293T (Invitrogen Corporation, Carlsbad, CA) was cultured in Dulbeco's modified Eagle's medium (Nacalai Tesque) supplemented with 10% fetal bovine serum (FBS), 100 U / ml penicillin and 100 μg / ml streptomycin. MT-4 cells were maintained in complete RPMI 1640 medium (Nacalai Tesque) supplemented with 10% FBS, 100 U / ml penicillin and 100 μg / ml streptomycin.
[0113] plasmid
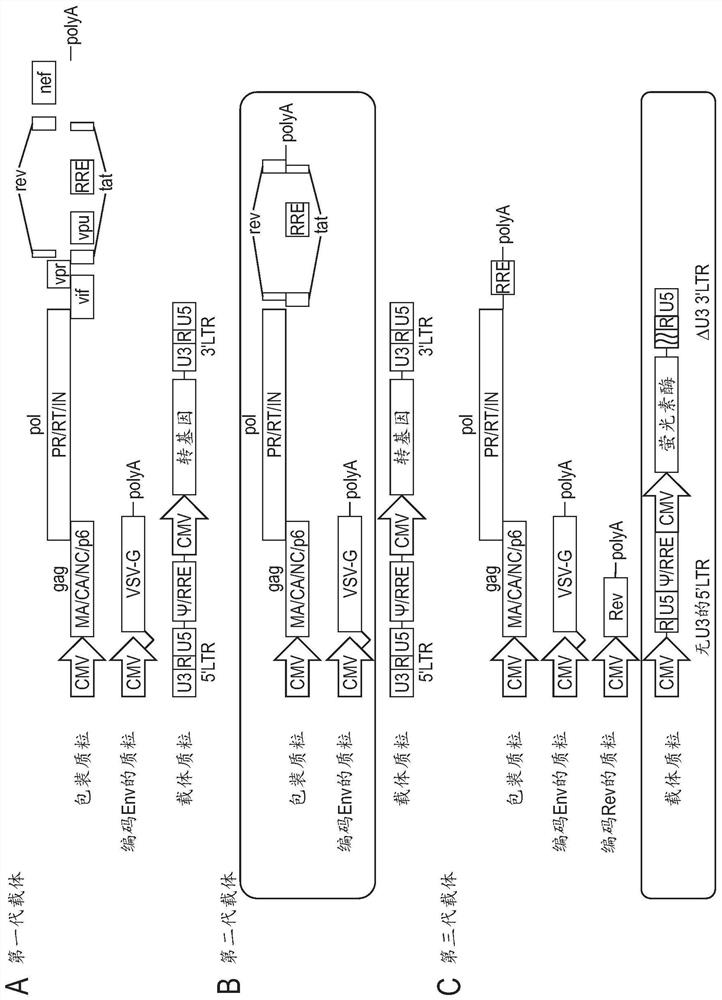
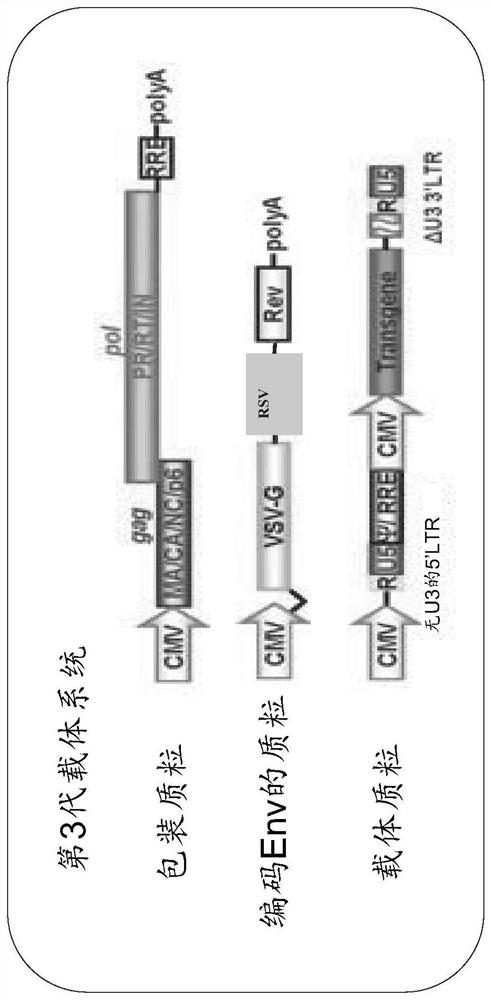
[0114] pCSII-CMV-MCS-IRES2-Bsd, pCAG-HIVgp, and pCMV-VSV-G-RSV-Rev were provided by Riken BRC through the Ministry of Education, Culture, Sports, Science and Technology's National Bioresource Project. To generate pCSII-CMV-luc-IRES2-Bsd, pCSII-CMV-MCS-IRES2-Bsd and pCMV-luc were digested with XhoI and NotI. Next, the XhoI-NotI fragment holding the firefly luciferase gene derived from pCMV-luc was inserted into the XhoI-NotI site of pCSII-CMV-MCS-IRES2-Bsd. The res...
Embodiment 2
[0137] [Example 2] Enhanced production of lentiviral vectors when HTLV-1Tax, Tat or NF-κB RelA are expressed individually or simultaneously
[0138] Next, using the third-generation lentiviral vector system pCAG-HIVgp, pCMV-VSV-G-RSV-Rev, pCSII-CMV-luc-IRES2-Bsd, using the luciferase activity in MT4 cells as an indicator, the following combinations The effect of increasing the production of lentiviral vectors caused by co-expression was studied (Fig. 7).
[0139] Figure 7-1 In means that Tax is co-expressed alone ( Figure 7-1 A), Tat alone co-expression ( Figure 7-1 B) Co-expression with RelA alone ( Figure 7-1 C) The effect on the yield of the third generation lentiviral vector.
[0140] Figure 7-2 In means co-expression in Tax, Tat and RelA ( Figure 7-2 A: Tax and Tat; Figure 7-2 B: Tax and RelA; Figure 7-2 C: Effects of Tat and RelA) on the yield of the third-generation lentiviral vector.
[0141] Figure 7-2 D shows the effect of the co-expression of Tax,...
Embodiment 3
[0143] [Example 3] The production of lentiviral vector is enhanced when Tat or NF-κB RelA is expressed alone or simultaneously
[0144] (1) In a collagen-coated 24-well plate, human embryonic kidney (Human Embryonic Kidney) (HEK) 293T cells were mixed with 0.5 mL of DMEM containing 10% bovine fetal serum at 3.0×10 5 cells / well for seeding and cell adhesion.
[0145](2) After 2 hours, mix the following plasmid with Opti-MEM (Gibco) 900 μL, add 1 μg / μL polyethyleneimine (Polyethyleneimine, PEI) 30 μL and incubate at room temperature for 30 minutes, and then drop into the cells .
[0146] (i) 0.25 μg pCAG-HIVgp
[0147] (ii) 0.125 μg pCMV-VSV-G-RSV-Rev
[0148] (iii) 0.375 μg pCSII-CMV-luc-IRES2-Bsd
[0149] (iv) 0.05 μg pSV2Tat or pSV2
[0150] (v) 0.025 µg of pRC / CMVRelA or pRC / CMV (manufactured by Invitrogen)
[0151] The structure of the pSV plasmid is as follows Figure 8 shown.
[0152] (3) After 24 hours, replace the culture medium.
[0153] (4) After 48 hours, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com