Application of AIBP in treating or inhibiting hemangioma
A hemangioma and overexpression technology, applied in the field of biomedicine, can solve problems such as lack of research, and achieve highly applicable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one, experimental material
[0024] 1. Matrigel was purchased from BD bioscience;
[0025] 2. rhAIBP was purchased from Beijing Yiqiao Shenzhou Company;
[0026] 3. Heparin was purchased from Sigma;
[0027] 4. DMEM was purchased from HyClone;
[0028] 5. F12K was purchased from Sciencell;
[0029] 6. Phosphate Buffered Saline (1×) was purchased from HyClone Company;
[0030] 7. Penicillin-Streptomycin Solution was purchased from HyClone Company;
[0031] 8. Fetal bovine serum was purchased from Sijiqing Company.
Embodiment 2
[0032] Embodiment two, experimental method
[0033] 1. Tube formation (tube formation experiment)
[0034] (1) Seed human hemangioma endothelial cells (HemECs) into six-well plates, 3×10 5 / Each well was cultured with DMEM / F12K medium, and after the cells adhered to the wall, they were starved overnight with serum-free basal medium.
[0035] (2) After the starvation was completed, the cell medium was changed, and the serum-free medium was replaced with DMEM / F12K medium, and the experimental group was treated with human recombinant protein AIBP (rhAIBP) for 24 hours.
[0036] (3) Take the melted matrigel, spread 50ul per well into a 96-well plate, place it in a 37-degree incubator and incubate for 30 minutes until it solidifies.
[0037] (4) Aspirate the cell solution in the 6-well plate, wash it with PBS, add trypsin to digest for 3 minutes, and stop the digestion. After centrifugation, use fresh DMEM / F12K to complete the culture to resuspend the cells, and use a hemocytome...
Embodiment 3
[0049] Example 3. Recombinant protein AIBP inhibits tube formation and migration of human hemangioma endothelial cells
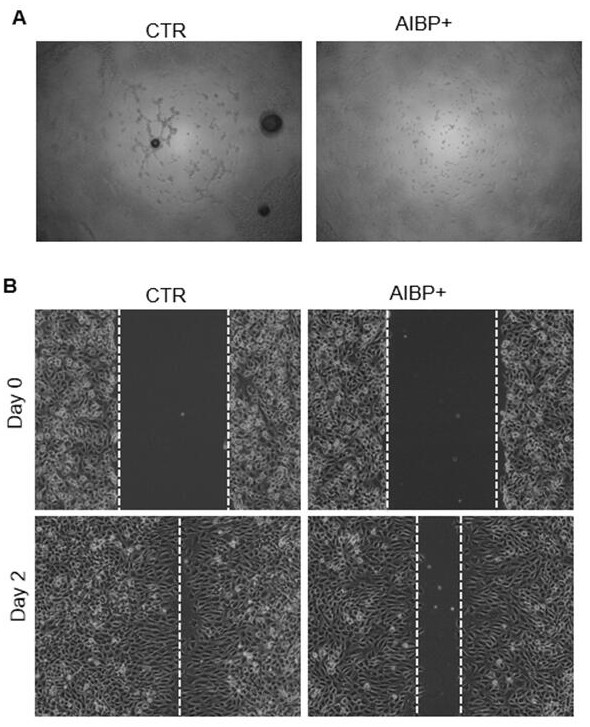
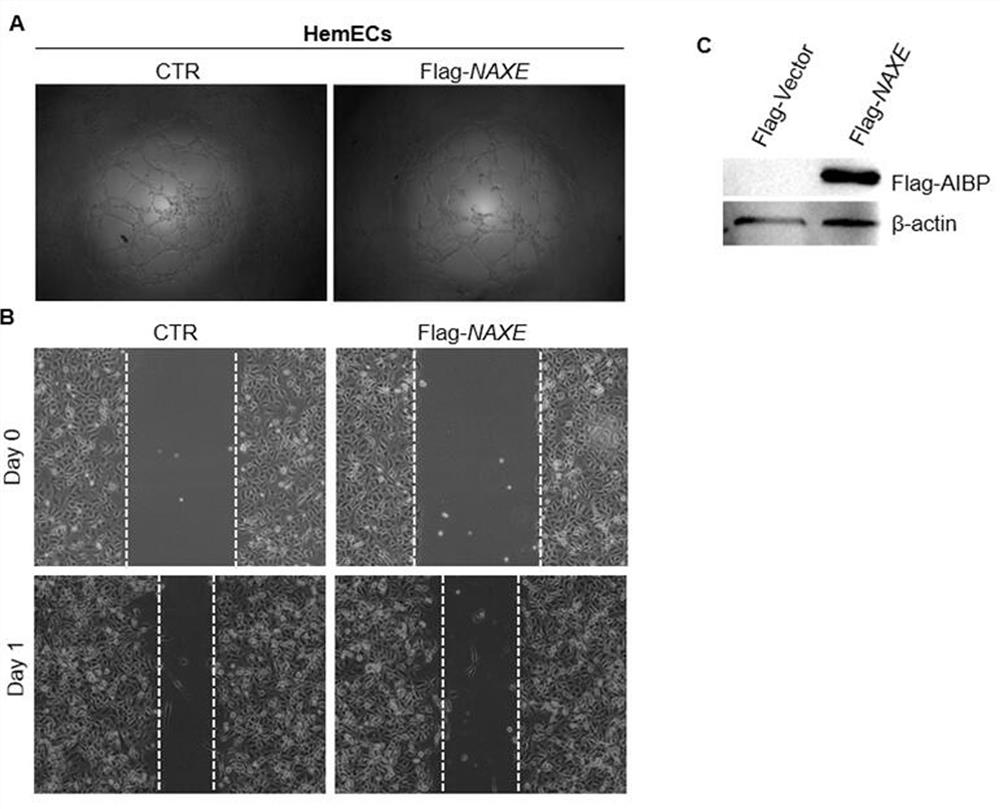
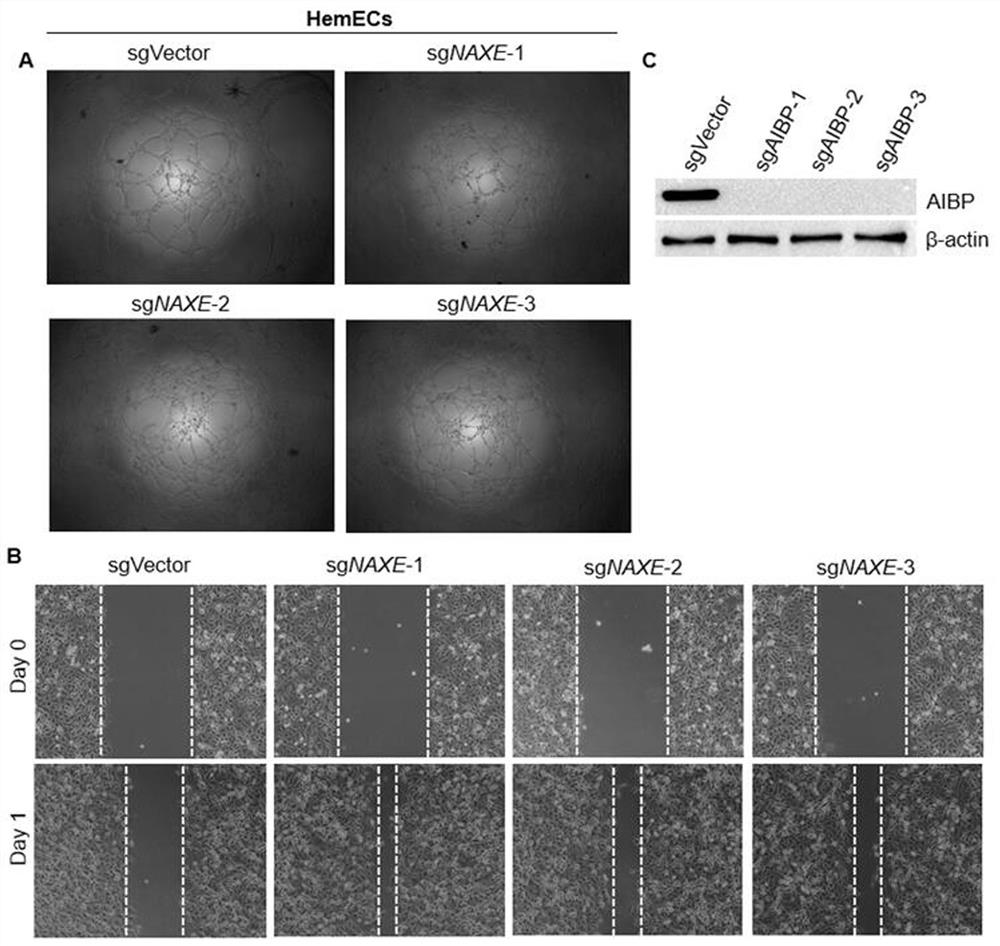
[0050] As a secreted protein, AIBP can inhibit retinal angiogenesis in zebrafish and mice. Hemangioma is a common tumor in infants, and its pathophysiological mechanism is to form new blood vessels. Therefore, the present invention proposes that AIBP may also play an important role in the occurrence of hemangioma .
[0051] In order to confirm this hypothesis, the present invention first uses recombinant human AIBP protein to conduct tube formation experiments in human hemangioma endothelial cells. The results of tube-forming experiments showed that compared with the control group (CTR), the tube-forming ability of hemangioendothelial cells HemECs in the secretory protein AIBP treatment group (AIBP+) was significantly weakened, as shown in figure 1 As shown in A. At the same time, the present invention detects whether AIBP affects the migration ability o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com