Derivative of vascular endothelial growth factor receptor antagonistic peptide F56 and application thereof
A technology of growth factor receptor and vascular endothelium, which is applied in the field of derivatives of vascular endothelial cell growth factor receptor antagonist peptide F56, can solve the problems of short half-life and achieve the effects of prolonged action time and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1, Preparation and Identification of F56 Modified Peptide
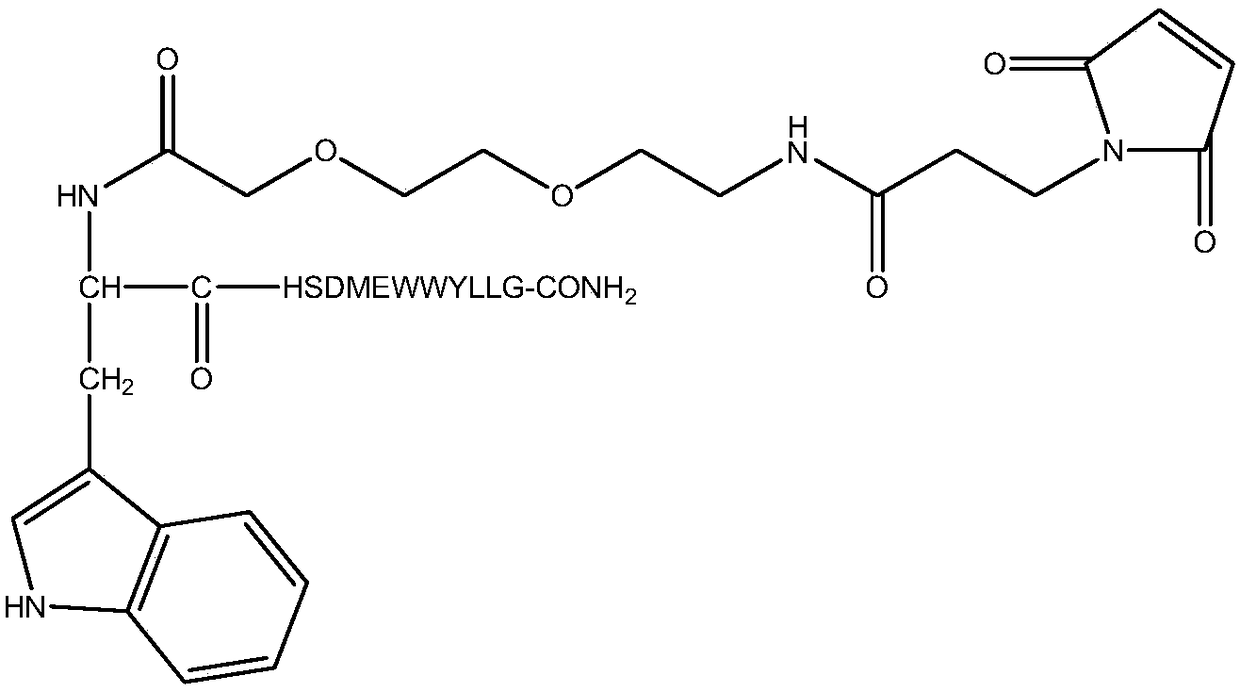
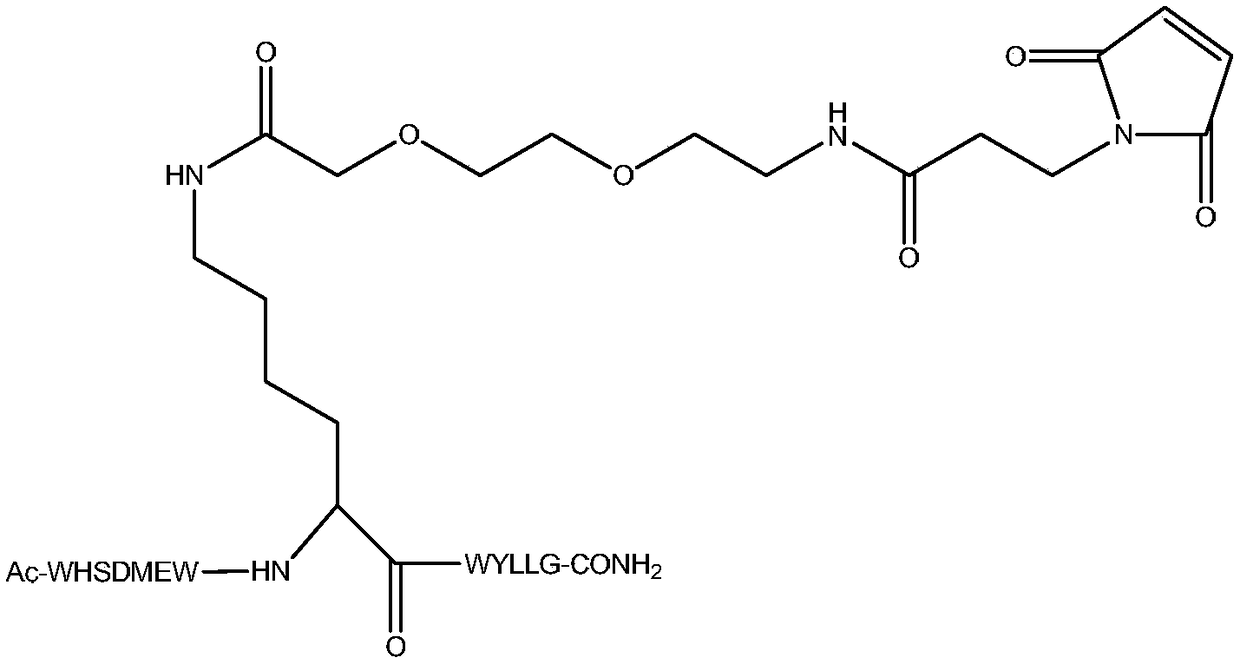
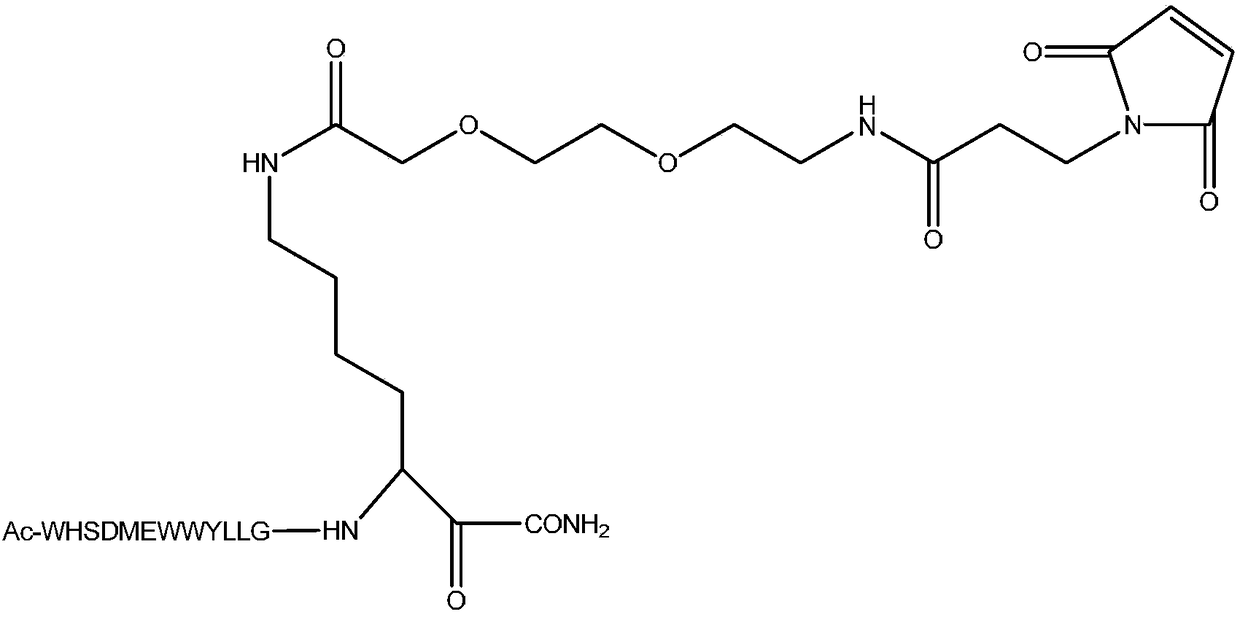
[0047] In this example, polypeptide F56 and F56 N-terminal modified peptide F56-AM ( figure 1 ), middle region modified peptide F56-BM ( figure 2 ) and carboxy-terminal modified peptide F56-CM ( image 3 ).
[0048] Modified peptide synthesis raw materials and related reagents, instruments:
[0049] 1. Resin: 2-Chlorotrityl Chloride Resin with a substitution degree of 1.03mmol / g (Tianjin Nankai Synthetic Technology Co., Ltd.);
[0050] 2. Amino acids: Fmoc-Gly-OH (Chengdu Chengnuo, >99%), Fmoc-Leu-OH (Chengdu Chengnuo, >99%), Fmoc-Tyr(Tbu)-OH (Chengdu Chengnuo, >99% ), Fmoc-Trp(Boc)-OH (Chengdu Chengnuo, >99%), Fmoc-Glu(OtBu)-OH (Chengdu Chengnuo, >99%), Fmoc-Met-OH (Chengdu Chengnuo, >99%) %), Fmoc-Asp(OtBu)-OH (Chengdu Chengnuo, >99%), Fmoc-Ser(tBu)-OH (Chengdu Chengnuo, >99%), Fmoc-His(Trt)-OH (Chengdu Chengnuo, >99%), Fmoc-His(Trt)-OH (Chengdu Chengnuo promise, >99%);
[0051] 3. Raw materia...
Embodiment 2
[0135] Example 2, F56 modified peptide can bind to human serum albumin in vitro
[0136] In this example, it was confirmed by in vitro experiments that the F56-AM, F56-BM, and F56-CM of the present invention prepared according to the method of Example 1 can bind to human serum albumin.
[0137] Experimental steps:
[0138] (1) Prepare 25% human serum albumin (3.79mM) solution: Weigh 0.25g of human serum albumin into a 1.5mL sterile Eppendorf tube, add 750μL of sterile phosphate buffer (100mM NaH 2 PO 4 / Na 2 HPO 4 , pH=7.4), vortexed to mix, and set the volume to 1.0 mL. No sonication was performed.
[0139] (2) Preparation of 10 mM polypeptide F56-AM / F56-BM / F56-CM-dimethylsulfoxide (DMSO) solution: dissolve the above polypeptide to 10 mM with DMSO.
[0140] (3) Preparation of polypeptide reaction solution: the above two solutions were mixed at a volume ratio of 9:1 (human serum albumin:modified peptide) to obtain a reaction solution corresponding to a molar ratio of 3:1...
Embodiment 3
[0151] Example 3, Study on the Activity of F56 Modified Peptides at Different Sites
[0152] 1. Tube Formation Experiment of Endothelial Cells
[0153] In this example, Matrigel was used to simulate the basement membrane of mammalian cells in vitro, and the differences in the formation of three-dimensional tubular structures on the extracellular matrix of human umbilical vein endothelial cells (HUVEC) with different treatments were observed.
[0154] Experimental steps of endothelial cell tubular structure formation:
[0155] (1) Pre-thaw Growth Factor Reduced Matrigel (BD company product number: 356230) on ice at 4°C, and pre-cool the 96-well plate and pipette tips at 4°C.
[0156] (2) Place the 96-well plate on ice, spread 50 μl of Matrigel on each well, and incubate at 37° C. for 30 minutes.
[0157] (3) Digest HUVEC primary cells, count, dilute to 10000 cells / 100 μl with serum-free medium, add final concentration of 0.1% BSA and 20ng / ml VEGF.
[0158] (4) F56-AM, F56-BM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com