Application of mulberone H in preparation of anti-Alzheimer's disease medicine
A technology of Alzheimer's and mulberry, which is applied in the field of medicine and can solve the problems such as no reports of butyrylcholinesterase inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
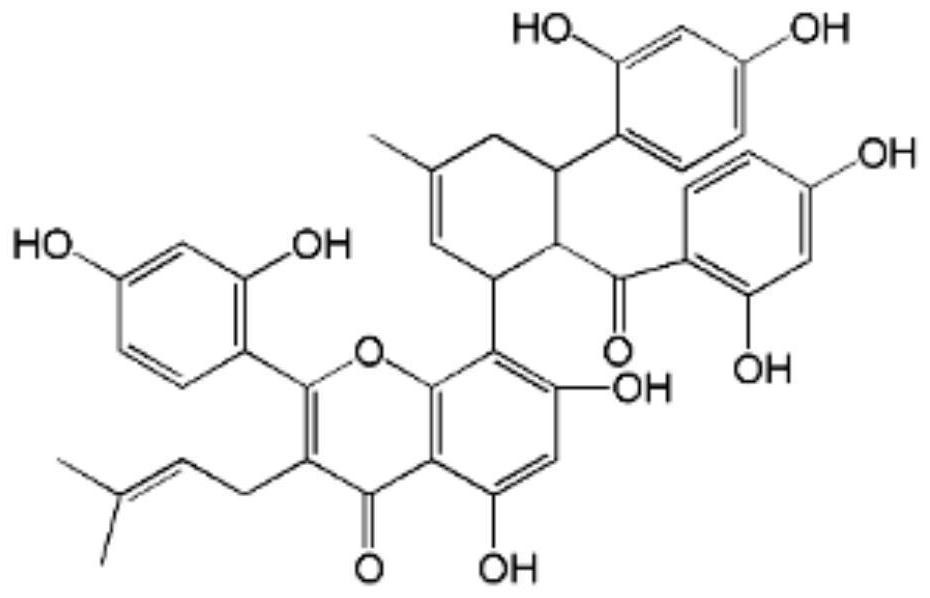
[0019] The applicant will Sulperone H (structural formula sees Figure 4 ) was compared with other flavonoid-related activities with similar structures, and the results are shown in Table 1.
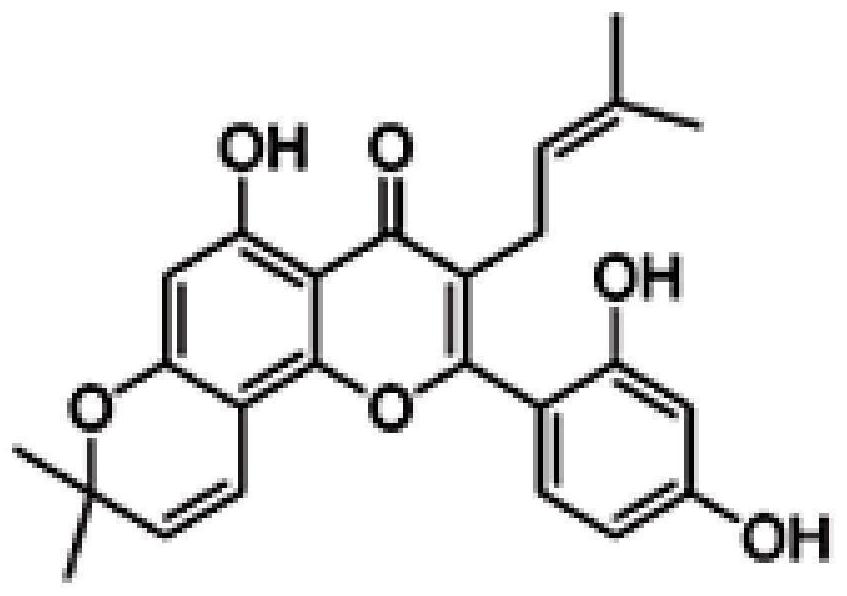
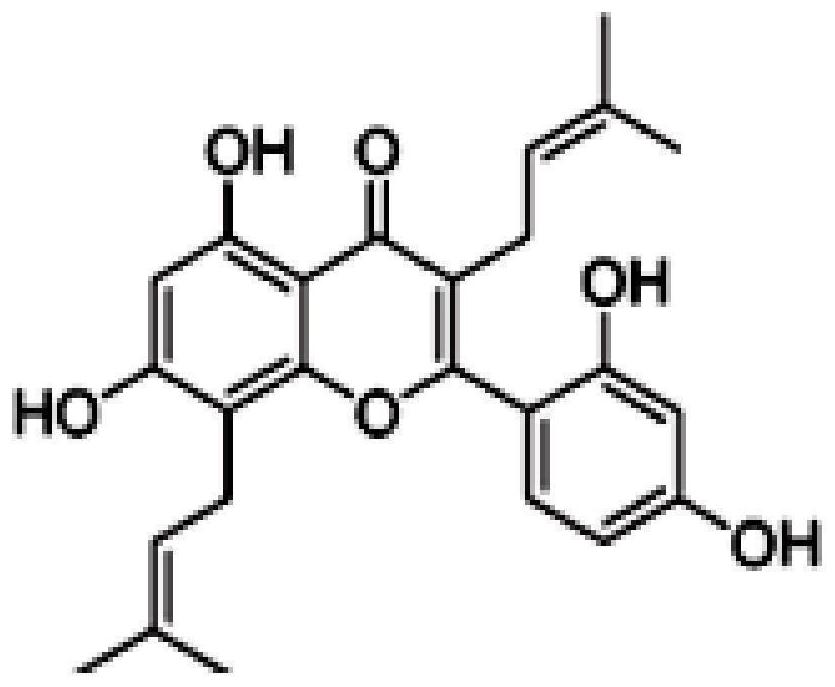
[0020] Concretely include: Morusin (Morusin, structural formula sees figure 1 ), mulperin C (Kuwanon C, structural formula see figure 2 ), Kuwanon G (Kuwanon G, structural formula see image 3 ).
[0021] IChE inhibition by the modified Ellman method 50 Value was tested, 40 μL of PBS (0.1M, PH 8.0), 20 μL of DTNB (5,5'-dithiobis(2-nitrobenzoic acid), 2mM), 10 μL of BChE (0.75u / mL) and 10 μL of samples with different concentrations were mixed, and then 20 μL of BTC (S-butyrylthiocholine iodide, 2 mM) was quickly added to start the reaction, and read at 405 nm using a microplate reader after 2 min.
[0022] Table 1. IC of prenylflavones with similar structures in Morus twig to BChE 50 value
[0023]
[0024] It can be seen from Table 1 that the inhibitory activity of mulperione ...
Embodiment 2
[0026] The in vitro inhibitory activity of BChE was tested by the modified Ellman method, 40 μL of PBS (0.1M, pH8.0), 20 μL of DTNB (5,5'-dithiobis(2-nitrobenzoic acid), 2mM) , 10 μL of BChE (0.75u / mL) and 10 μL of different concentrations of samples were mixed, and then quickly added 20 μL of BTC (S-butyrylthiocholine iodide, 2mM) to start the reaction. After 2 minutes, use a microplate reader at 405nm reading. The inhibition rate was calculated according to the following formula:
[0027]
[0028] Among them, A1 is the absorbance value of the sample group, A2 is the absorbance value of the blank without enzyme group, and A0 is the negative absorbance value without adding the sample. All experiments were repeated three times and the mean and standard deviation were calculated.
[0029] Experimental results (table 2): by in vitro inhibition of butyrylcholinesterase activity assay found that mulperione H has a good inhibitory activity to butyrylcholinesterase, and with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com