Application of Htra2 gene expression inhibitor in prevention of acquired sensorineural deafness
A technology for sensorineural hearing loss and gene expression, applied in the field of genetic engineering, can solve the problems of cell apoptosis, research on the ototoxicity of antibiotics that have not yet been found, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preventive effect of SpCas9 system on deafness caused by aminoglycoside ototoxicity.
[0026] 1. Design AAV-CRISPR / SpCas9 treatment system.
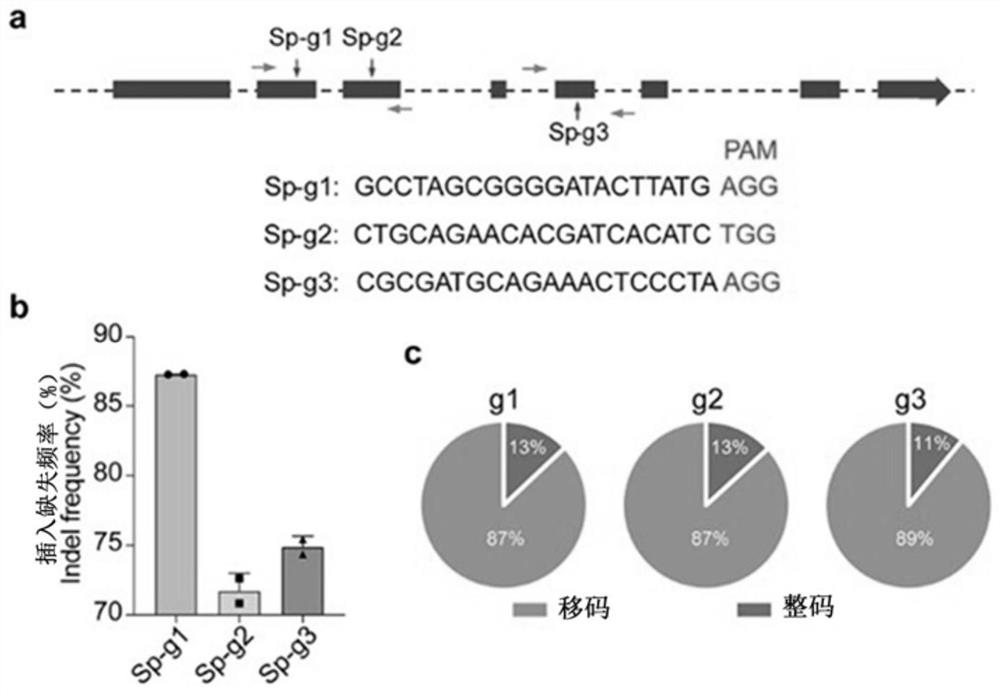
[0027] figure 1 It is the construction result diagram of the CRISPR / SpCas9 therapeutic system designed according to the mouse Htra2 gene sequence in Example 1. gRNA design diagram ( figure 1 Panel a in ) shows the targeting sequences of the three gRNAs, which are
[0028] Sp-g1: GCCTAGCGGGGATACTTATG (SEQ ID NO: 1);
[0029] Sp-g2: CTGCAGAACACGATCACATC (SEQ ID NO: 2);
[0030] Sp-g3: CGCGATGCAGAAACTCCCTA (SEQ ID NO: 3).
[0031] figure 1 Figure b in the figure is the in vitro editing efficiency results of three gRNAs; figure 1 Figure c in the figure is the proportion of three gRNAs causing In-frame and Frameshift mutations.
[0032] figure 1 Figure a in the figure also shows PAM (protospacer adjacent motif). CRISPR targeting specificity is determined by two parts, one part is the base pairing between the RNA chimera and t...
Embodiment 2
[0039] Preventive effect of SaCas9 system on deafness caused by aminoglycoside ototoxicity.
[0040] 1. Design AAV-CRISPR / SaCas9 treatment system.
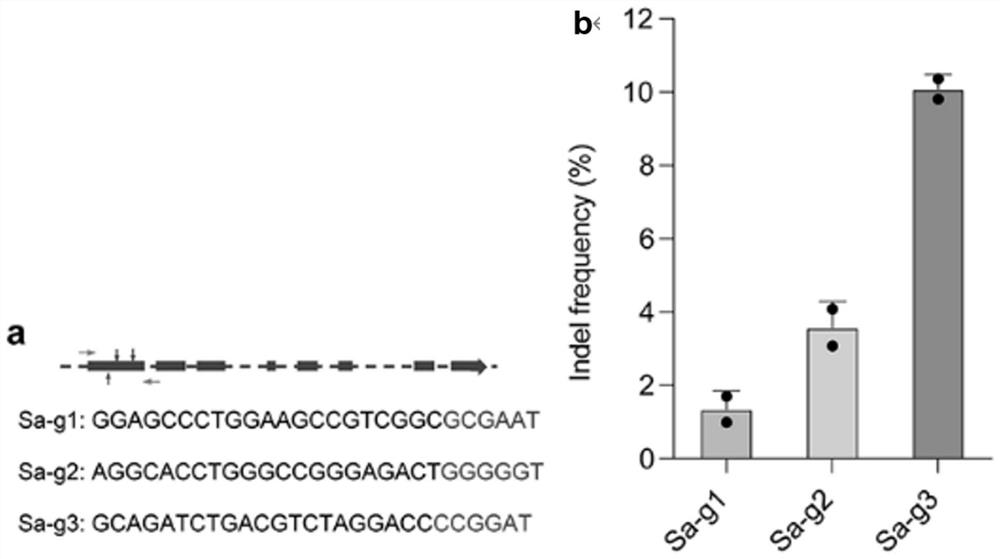
[0041] image 3 It is the construction result diagram of the AAV-CRISPR / SaCas9 therapeutic system designed according to the mouse Htra2 gene sequence in Example 2. image 3 Figure a in the figure is a gRNA design diagram, which shows the targeting sequences of three gRNAs;
[0042] Sa-g1: GGAGCCCTGGAAGCCGTCGGC (SEQ ID NO: 4);
[0043] Sa-g2: AGGCACCTGGGCCGGGAGACT (SEQ ID NO: 5);
[0044] Sa-g3: GCAGATCTGACGTCTAGGACC (SEQ ID NO: 6). The sequence ends with PAM.
[0045] image 3 Figure b in the figure is the in vitro editing efficiency results of three gRNAs, and Sa-g3 with the highest editing efficiency was selected for subsequent in vivo experiments.
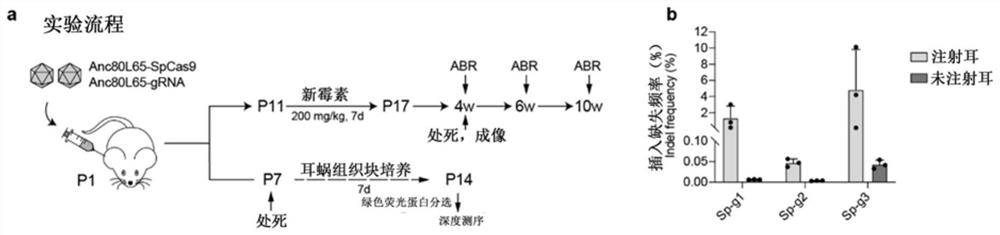
[0046] 2. The AAV-CRISPR / SaCas9 treatment system was introduced into the middle stage of the inner ear by microinjection in P1 of neonatal mice. Neomycin injury began at P11 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com