Method for preparing beta-carbonyl sulfone compound by using half-sandwich iridium complex
A technology of iridium complexes and carbonyl sulfones, which is applied in the field of preparation of β-carbonyl sulfone compounds, can solve the problems of multi-step preparation of substrates, harsh reaction conditions, and sensitive catalysts, and achieves a simple and green preparation method, mild reaction conditions, and side effects. The effect of less product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
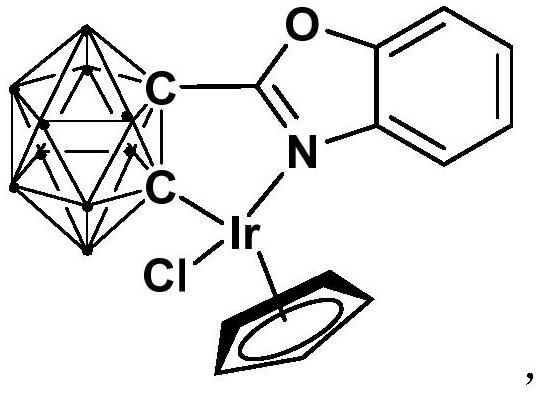
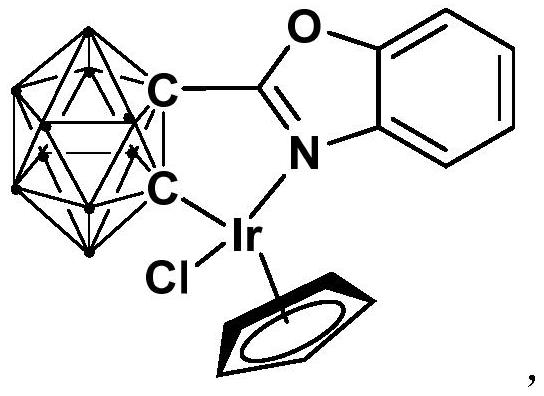
[0029] Synthesis of semi-sandwich iridium complex Ir containing ortho carboryl benzoxazole structure:
[0030]
[0031] At -78°C, n-BuLi (1.6M) n-hexane solution (1.6mmol) was slowly added dropwise to the ortho carborane o-C 2 B 10 h 12 (0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added bromobenzoxazole (0.64mmol), and continued to react at room temperature for 6 hours. Then the binuclear iridium compound [Cp*IrCl 2 ] 2 (256.0 mg, 0.32 mmol) was added to the reaction system for an additional 3 hours. After the reaction, stand and filter, and dry the solvent under reduced pressure. The obtained crude product is separated by column chromatography (volume ratio, petroleum ether / tetrahydrofuran = 6:1), and the red target product is obtained, which is iridium (III) Complex Ir (81% yield).
[0032] 1 H NMR (400MHz, CDCl 3 ,25℃): δ=7.88(d, J=7.0Hz, 1H), 7.72(t, J=...
Embodiment 2
[0035] Synthesis of β-carbonyl sulfone compounds catalyzed by iridium(III) complexes:
[0036]
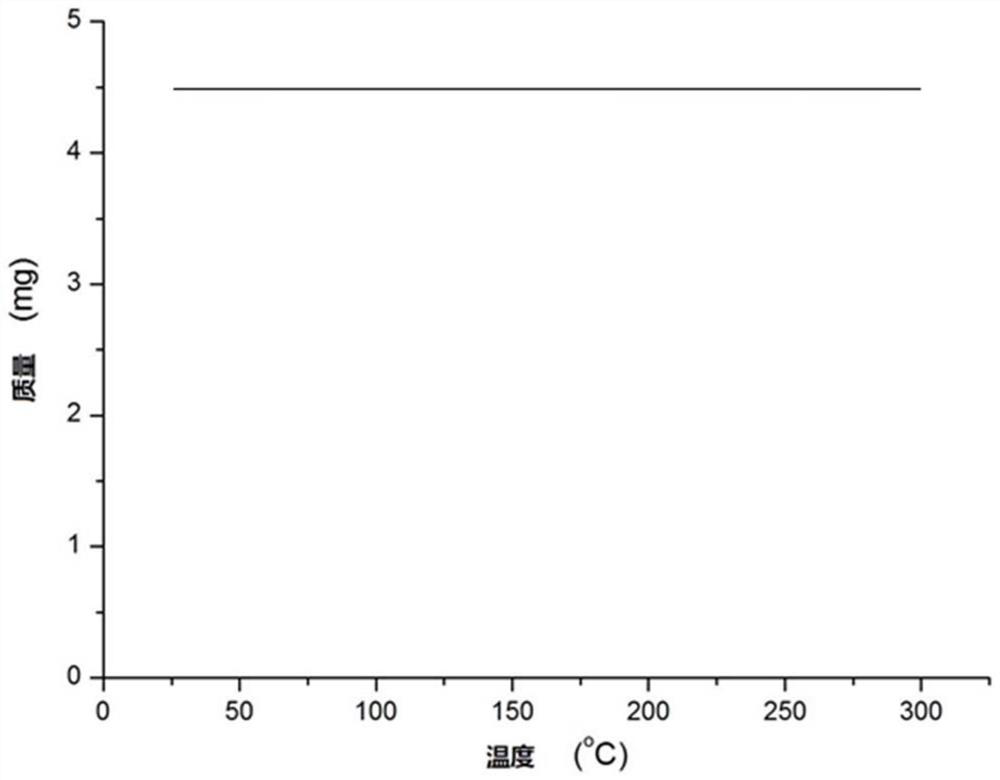
[0037] The iridium (III) complex prepared in Example 1 was used as a catalyst to catalyze the reaction of acetophenone and benzenesulfonyl chloride: in acetophenone (1 mmol), a trivalent iridium complex (0.001 mmol) in toluene, then add benzenesulfonyl chloride (1mmol) and K 2 CO 3 (1.2mmol), reacted at room temperature for 150 minutes, after the end, the concentrated reaction solution was directly separated by silica gel column chromatography, dried until the mass remained constant, and the corresponding β-carbonyl sulfone compound C 14 h 12 o 3 S (yield 89%), 1 H NMR (CDCl 3 ,500MHz): δ7.95(d,2H,J=8.0Hz),7.90(d,2H,J=7.5Hz),7.67(t,1H,J=7.5Hz),7.60(t,1H,J= 7.0Hz), 7.55(t, 2H, J=7.5Hz), 7.45(t, 2H, J=7.7Hz), 4.77(s, 2H). Elemental analysis: C 64.60, H 4.65 (theoretical); C 64.66, H 4.69 (actual).
Embodiment 3
[0039]Synthesis of β-carbonyl sulfone compounds catalyzed by iridium(III) complexes:
[0040]
[0041] The iridium (III) complex prepared in Example 1 is used as a catalyst to catalyze the reaction of acetophenone and 4-methylbenzenesulfonyl chloride: add trivalent iridium containing an ortho carborane structure to acetophenone (1 mmol) Complex (0.003mmol) in toluene solution, then add 4-methylbenzenesulfonyl chloride (1mmol) and K 2 CO 3 (1.3mmol), react at room temperature for 200 minutes, after the end, the concentrated reaction solution is directly separated by silica gel column chromatography, dried until the mass remains unchanged, and the corresponding β-carbonyl sulfone compound C 15 h 14 OS (93% yield), 1 H NMR (CDCl 3 ,500MHz): δ7.96(dd,2H,J=8.5,1.5Hz),7.78(d,2H,J=8.0Hz),7.65(t,1H,J=7.0Hz),7.49(dd,2H, J=8.0, 7.0Hz), 7.36(d, 2H, J=8.0Hz), 4.75(s, 2H), 2.45(s, 3H). Elemental analysis: C 65.67, H 5.14 (theoretical); C 65.75, H 5.20 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com