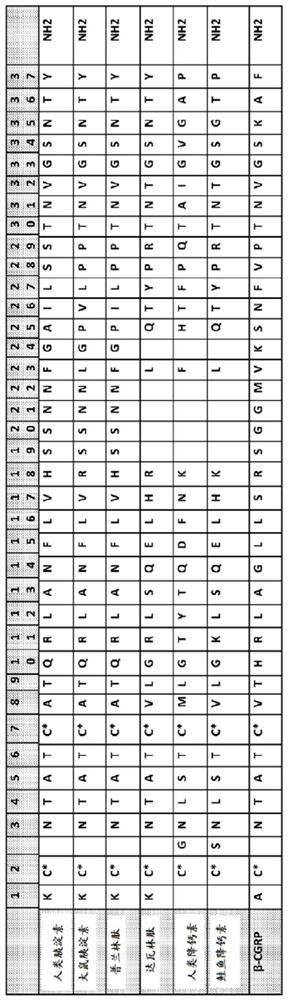
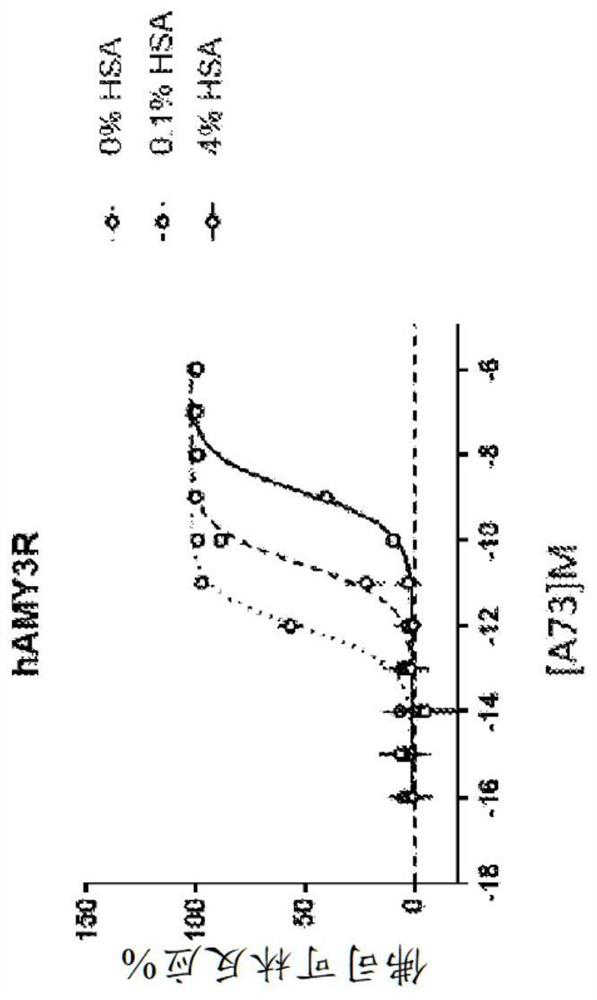
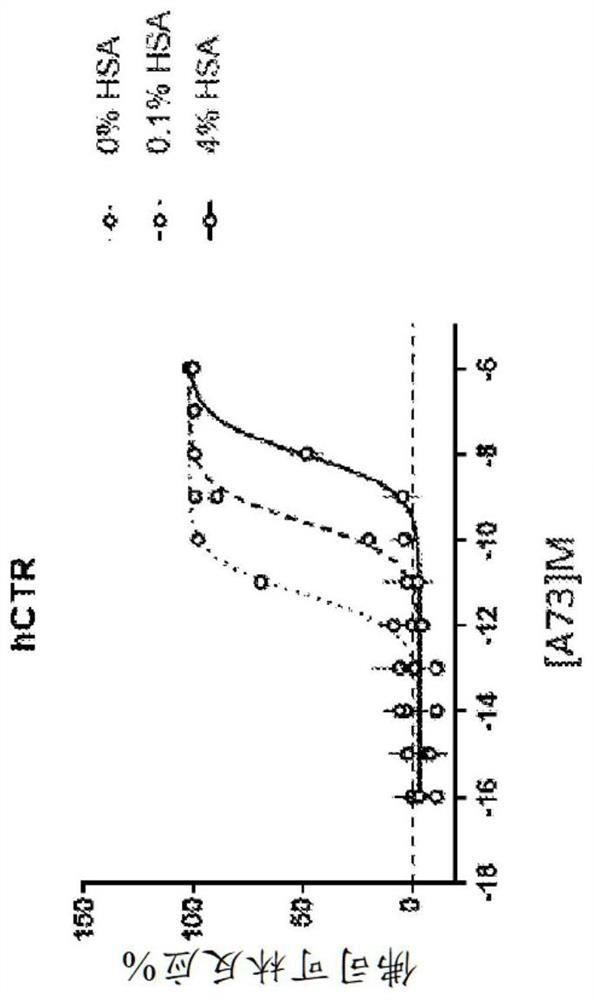
Human amylin analog polypeptides and methods of use
An optional amino acid technology, applied in the field of amylin analog polypeptides, treating metabolic diseases or conditions such as type 1 and type 2 diabetes and providing weight loss, can solve the problem that pramlintide cannot be mixed with insulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[1004] Example 1: Generation of Amylin Analog Polypeptides
[1005]Using the Fmoc strategy with N-[(dimethylamino)-1H-1,2,3-triazolo-[4 ,5-b]pyridin-1-ylmethylene]-N-methylmethylammonium hexafluorophosphate N-oxide (HATU) or 2-(6-chloro-1-H-benzotriazole-1 -base)-1,1,3,3-tetramethylammonium hexafluorophosphate (HCTU) activation (amino acid 5-fold molar excess), synthesized in N,N-dimethylformamide (DMF) as shown in Table 3 The amylin analog polypeptide of the present invention provided in , and N'N-diisopropylethylamine (DIEA) was used as the base. 20% piperidine / DMF solution was used for Fmoc deprotection. The resin used was Rink Amide MBHA LL (Novabiochem) with a loading of (0.30-0.40) mmol / g on a (20-400) μmol scale.
[1006] Final deprotection and cleavage of the peptide from the solid support was performed by treating the resin with (92.5% TFA, 2.5% phenol, 2.5% water and 2.5% triisopropylsilane) for 2-3 hours. The cleaved peptide was precipitated using cold diethyl e...
Embodiment 2
[1008] Example 2: Purification and Characterization of Amylin Analog Polypeptides, i.e. Linear Polypeptides Without Any Lipophilic Substituents and Optional Spacers
[1009] The purified product was lyophilized and analyzed by ESI-LC / MS and analytical HPLC and proved to be pure (>98%). The mass results were all in agreement with the calculated values.
[1010] Performed via C18 HPLC and LC / MS analysis (Acquity SQD Waters Corp, Milford, MA) with UV detection provided by dual absorbance signals at 215 nm and 280 nm using one of Method A, Method B, Method C or Method D Characterization of Peptide Analogs.
[1011] Method A, LC / MS conditions: using Phenomenex UPLC Aeris TM Peptide XB C18 35 column, 1.7pm, 2.1×100mm or ACQUiTY BEH300 or BEH130 CT8 column, 1.77pm, 2.1×100mm, using 5-65% acetonitrile / water with 0.05% TFA in 30 minutes, flow rate 0.5mL / min, λ-215nm, 280nm.
[1012] Method B, C18 HPLC conditions: On Acquity BEH130, C18 column, 1.7 μm, 100 × 2.10 mm column at 25 °C...
Embodiment 3
[1017] Embodiment 3: synthetic amylin analog polypeptide intermediate
[1018] Synthesis of polypeptides with modifications at e.g. D-Lys16 or L-Lys16 positions (compounds B2, A129, B4 and B8)
[1019] After completion of the synthesis of linear polypeptides as described in Example 1, the resin was washed with dichloromethane (DCM) and dried under vacuum for 30 minutes. For analogs containing allyloxycarbonyl protecting groups, via Pd(PPh 3 ) 3 Its removal is facilitated in a solution of (chloroform / acetic acid / n-methyl-morpholine, 37:2:1). The resulting deprotected resin was washed with 2% sodium diethyldithiodicarbamate trihydrate / DMF (6 x 30 sec), 2% DIEA / DMF (6 x 30 sec) and finally DMF (6 x 30 sec). The extension of the spacer subregions was performed with the manual addition of each building block under preactivation conditions in a stepwise fashion. To 1 ml of a 200 mmol solution of Fmoc-γGlu-(OH)-OtBu in DMF was added 0.5 ml DIEA (800 mmol), followed by 0.5 ml HCTU...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com