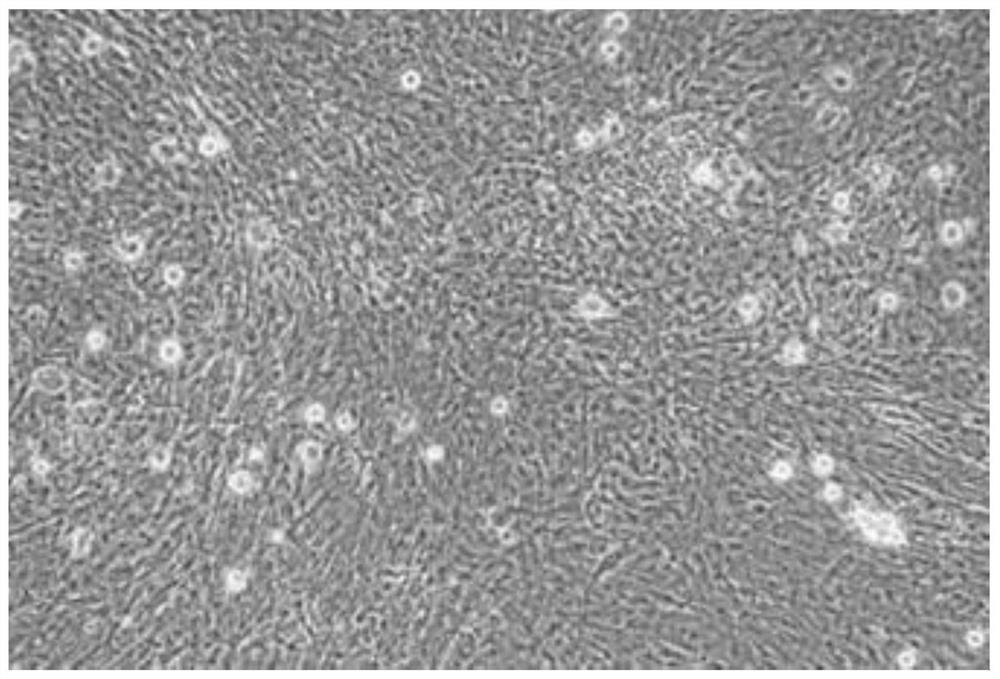
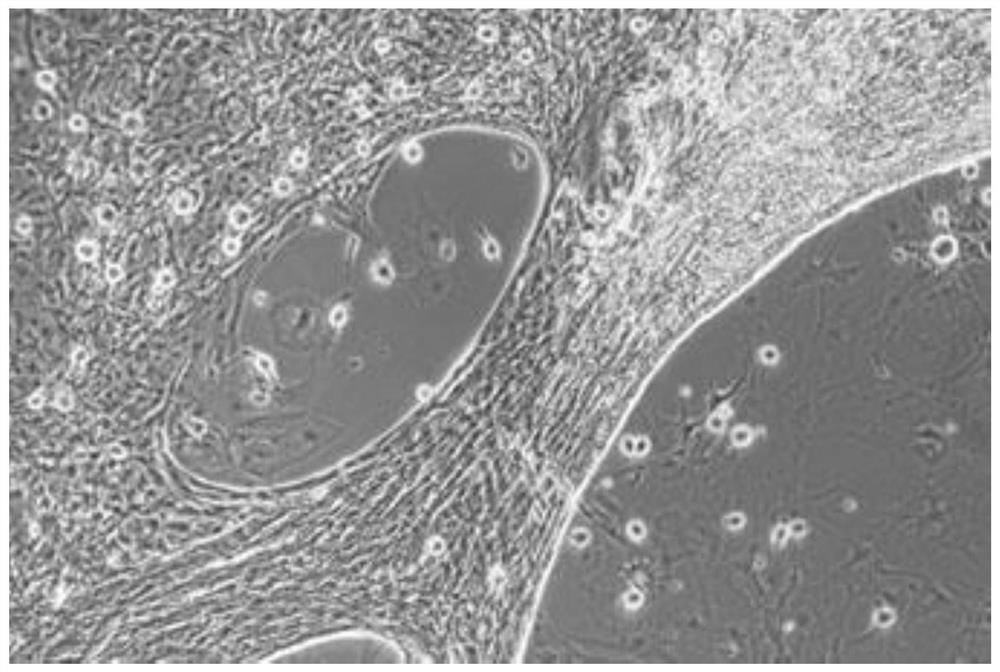
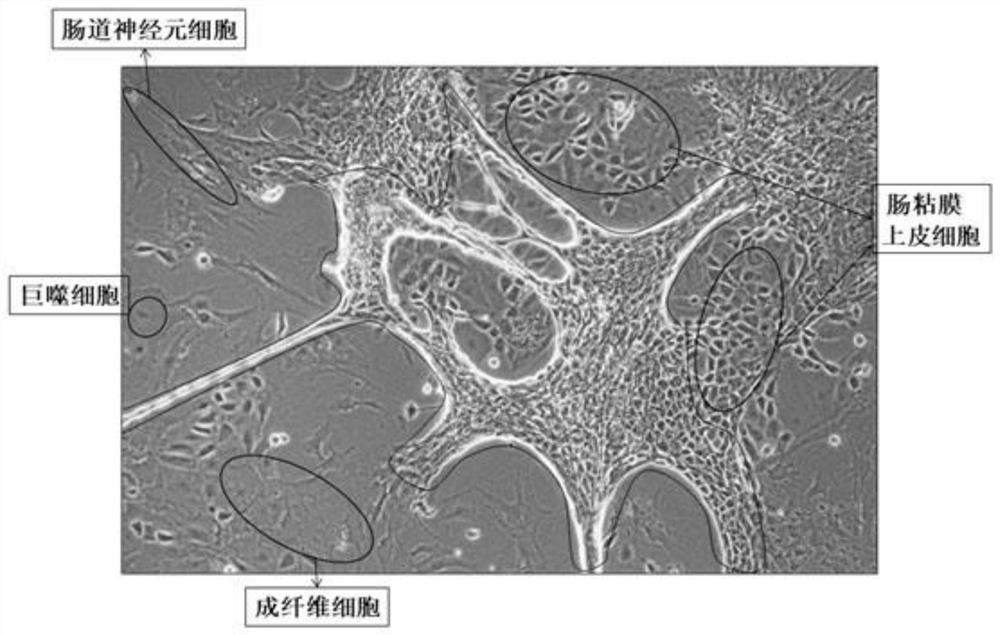
Method for separating and culturing mouse small intestine organoid capable of peristalsis in vitro
A technology of organoids and small intestines, which is applied in the field of cultivating peristaltic mouse small intestine organoids and in vitro separation, which can solve the problems of complicated operation, difficult cultivation, and inability to observe for a long time, and achieve the effect of simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Separation method
[0040] Step 1: Take three c57BL mice one week after birth, kill them by cervical dislocation, and sterilize them by immersing them in 70% alcohol for 1 min;
[0041] Step 2: Dissect the mouse in the ultra-clean workbench, take out all the large intestine and small intestine, put them in a 100mm Petri dish filled with PBS, and remove the large intestine;
[0042] Step 3: Using ophthalmic scissors, cut the small intestine longitudinally along the small intestine;
[0043] Step 4: Use the curved convex side of the curved ophthalmic forceps to gently scrape off the residues inside the small intestine and most of the small intestinal mucosal epithelium;
[0044] Step 5: Wash 2-3 times with 1xPBS buffer. After removing the washing supernatant, use ophthalmic scissors to cut the small intestine tissue into a size less than 1mm 3 Tissue fragments, and transferred to a 50mL centrifuge tube;
[0045] Step 6: After washing twice with 1xPBS, centrifuge wi...
Embodiment 2
[0063] 1. Separation method
[0064] Step 1: Take three c57BL mice one week after birth, kill them by cervical dislocation, and sterilize them by immersing them in 70% alcohol for 1 min;
[0065] Step 2: Dissect the mouse in the ultra-clean workbench, take out all the large intestine and small intestine, put them in a 100mm Petri dish filled with PBS, and remove the large intestine;
[0066] Step 3: Using ophthalmic scissors, cut the small intestine longitudinally along the small intestine;
[0067] Step 4: Use the curved convex side of the curved ophthalmic forceps to gently scrape off the residues inside the small intestine and most of the small intestinal mucosal epithelium;
[0068] Step 5: Wash 2-3 times with 1xPBS buffer. After removing the washing supernatant, use ophthalmic scissors to cut the small intestine tissue into a size less than 1mm 3 Tissue fragments, and transferred to a 50mL centrifuge tube;
[0069] Step 6: After washing twice with 1xPBS, centrifuge wi...
Embodiment 3
[0087] 1. Separation method
[0088] Step 1: Take three c57BL mice one week after birth, kill them by cervical dislocation, and sterilize them by immersing them in 70% alcohol for 1 min;
[0089] Step 2: Dissect the mouse in the ultra-clean workbench, take out all the large intestine and small intestine, put them in a 100mm Petri dish filled with PBS, and remove the large intestine;
[0090] Step 3: Using ophthalmic scissors, cut the small intestine longitudinally along the small intestine;
[0091] Step 4: Use the curved convex side of the curved ophthalmic forceps to gently scrape off the residues inside the small intestine and most of the small intestinal mucosal epithelium;
[0092] Step 5: Wash 2-3 times with 1xPBS buffer. After removing the washing supernatant, use ophthalmic scissors to cut the small intestine tissue into a size less than 1mm 3 Tissue fragments, and transferred to a 50mL centrifuge tube;
[0093] Step 6: After washing twice with 1xPBS, centrifuge wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com