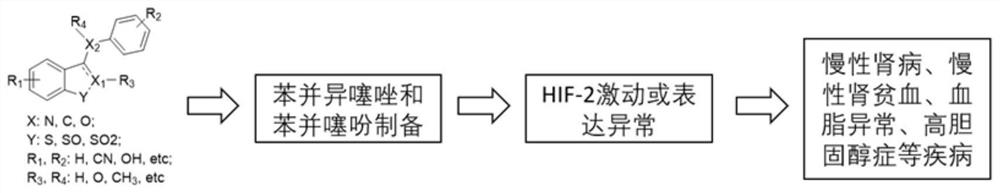
Preparation method and medical application of benzisothiazole and benzothiophene
A technology of benzisothiazole and benzothiophene is applied in the field of preparation of benzisothiazole and benzothiophene or their pharmaceutically acceptable salts, benzisothiazole and benzothiophene, and can solve the problem of increasing renal anemia Patients, high risk of cardiovascular disease and mortality, high price of rhEPO, to achieve good industrialization prospects, enhanced activity, good agonistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
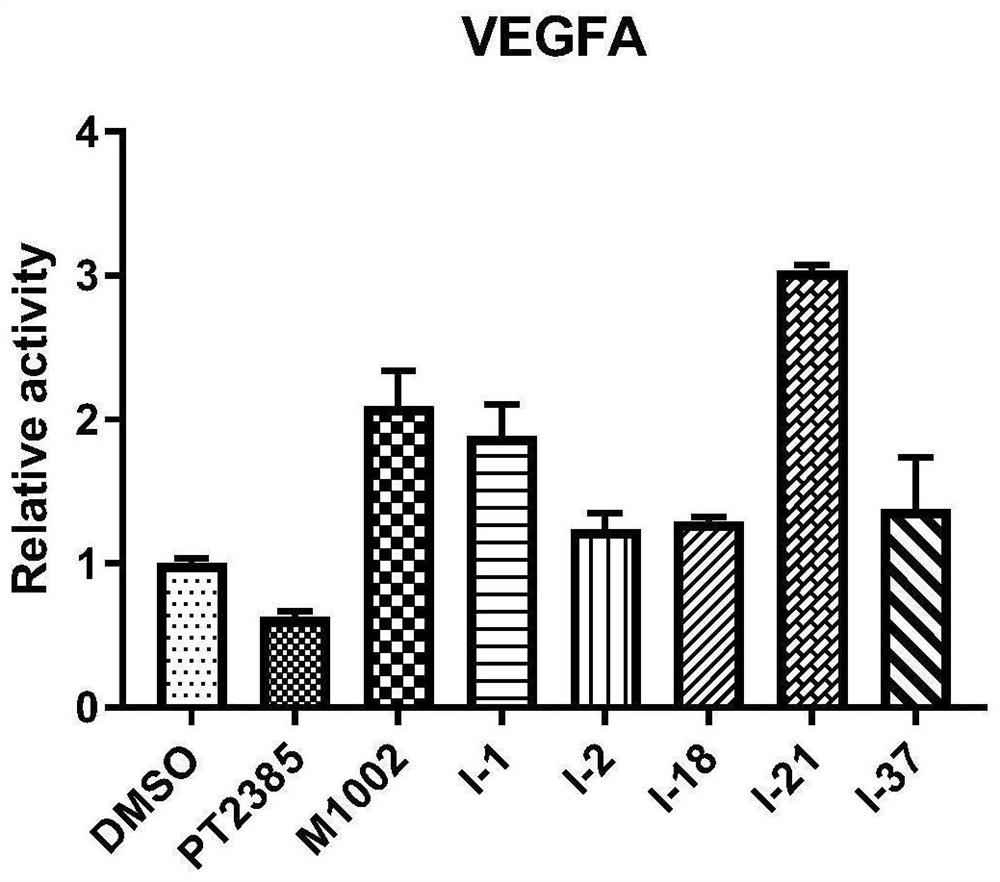
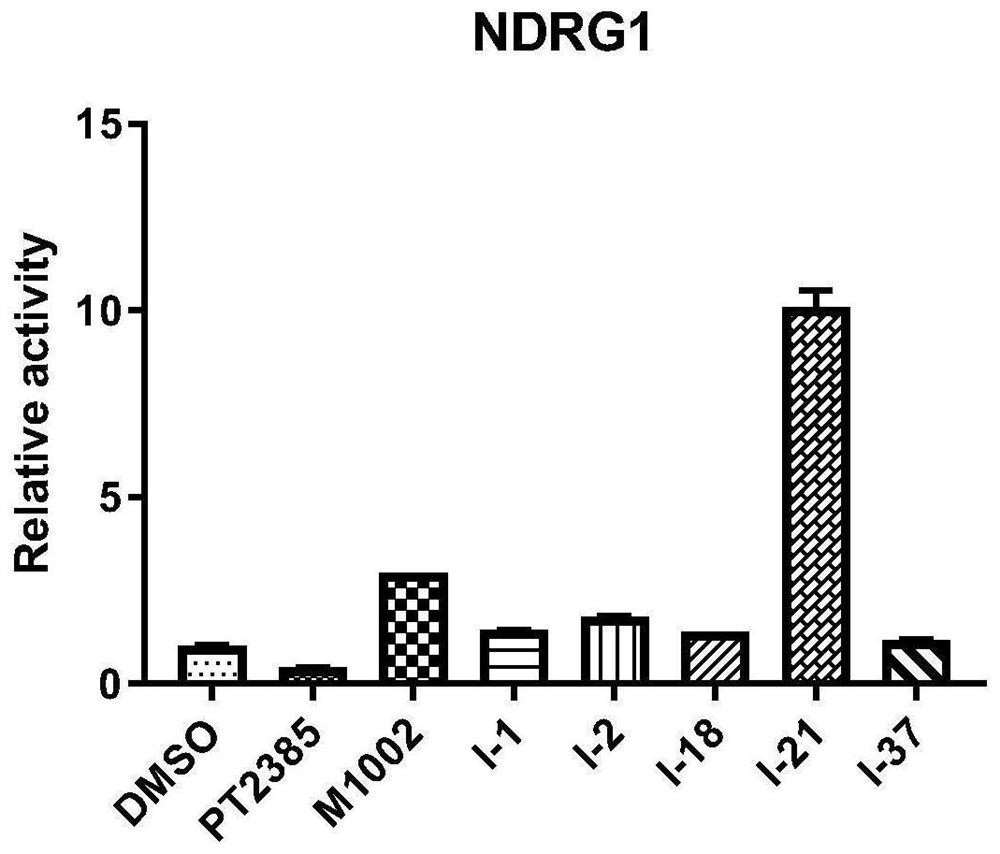
Image
Examples
Embodiment 1
[0099] Preparation of (I-1) of 3-((3,5-bis(trifluoromethyl)phenyl)(phenyl)amino)benzo[d]isothiazole 1,1-dioxide:
[0100]
[0101] Synthesis of 1a: Palladium acetate (11mg, 0.05mmol) and DPPE were dissolved in toluene (39mg, 0.1mmol), stirred at 110°C for ten minutes, iodobenzene (204mg, 1mmol) and 3,5-ditrifluoromethyl A mixed solution of aniline (229mg, 1mmol) dissolved in toluene (5mL) was added, and finally sodium methoxide (59.4mg, 1.1mmol) was added, and reacted for 24h; after the reaction was completed, cooled to room temperature, concentrated under reduced pressure, and column chromatography (PE: EtOAc=120:1) gave an oily solid in 70% yield. 1 H NMR (500MHz, CDCl 3 )δ(ppm):7.56(s,2H),7.49(s,1H),7.40-7.33(m,2H),7.21-7.01(m,3H).
[0102]
[0103] Synthesis of I-1: Refer to Method 1, using saccharin and 1a as raw materials to obtain a yellow solid with a yield of 45%. 1 H NMR (500MHz, CDCl 3 )δ(ppm):8.50-8.43(m,1H),8.31(s,2H),8.02(m,1H),7.96-7.89(m,2H),7.49(s,1H...
Embodiment 2
[0105] 3-((3,5-Bis(trifluoromethyl)phenyl)(cyclopropyl)amino)benzo[d]isothiazole 1,1-dioxide (I-5) Preparation:
[0106]
[0107] Synthesis of 2a: 2b (200mg, 1.2mmol) and 3,5-bistrifluoromethylaniline (229mg, 1mmol) were dissolved in methanol (4mL) and acetic acid (5mL) was added, stirred at 80°C for 2.5h, and cyanide was added at room temperature Sodium borohydride (126mg, 2mmol), stirred at 80°C for 3h; after the reaction was completed, cooled to room temperature, quenched with saturated sodium bicarbonate solution, then extracted with ethyl acetate, the organic phase was washed with saturated brine, anhydrous sulfuric acid It was dried over sodium, filtered, and the filtrate was concentrated under reduced pressure, followed by column chromatography (PE:EtOAc=120:1) to obtain a yellow oily solid with a yield of 55%. 1 H NMR (CDCl 3 ,500MHz)δ(ppm):7.25(s,1H),7.05(s,2H),2.25(m,1H),0.90-0.80(m,2H),0.69-0.60(m,2H).
[0108]
[0109] Synthesis of I-2: refer to method 1, u...
Embodiment 3
[0111] Preparation of 5-((1,1-dioxybenzo[d]isothiazol-3-yl)amino)dimethylisophthalate (I-3):
[0112]
[0113] Synthesis of I-3: Refer to Method 1, using 3a and saccharin as raw materials to obtain a white solid with a yield of 40%. 1H NMR (500MHz, DMSO-d 6 )δ(ppm):11.16(s,1H),8.82(s,2H),8.50(d,J=7.5Hz,1H),8.33(s,1H),8.13(d,J=7.5Hz,1H) ,7.95(m,2H),3.95(s,6H).13C NMR(126MHz,DMSO-d 6 )δ(ppm): 165.49×2, 157.52, 140.89, 139.50, 134.41, 134.01, 131.44×2, 128.59, 126.56, 126.35×2, 124.18, 122.08, 53.22×2.HRMS(ESI):m / z[M+H] + calcd for C 17 h 15 N 2 o 6 S 375.0651; found 375.0644.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com