Application of rhfgf-21 in preparation of medicine for treating dry eye syndrome
A technology of rhfgf-21, 1. rhfgf-21 is applied in the application field of rhFGF-21 in the preparation of medicaments for treating dry eye, can solve problems such as adverse reactions, and achieve the effects of reducing signs of dry eye and promoting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0047]
[0048]
[0055] The bagged phosphate buffered saline powder was dissolved in 2L of purified water and mixed thoroughly to obtain a 0.01M PBS buffer.
[0057] Add 10 g of paraformaldehyde powder to the PBS solution, dilute to 250 ml, and store in an airtight container. join 1
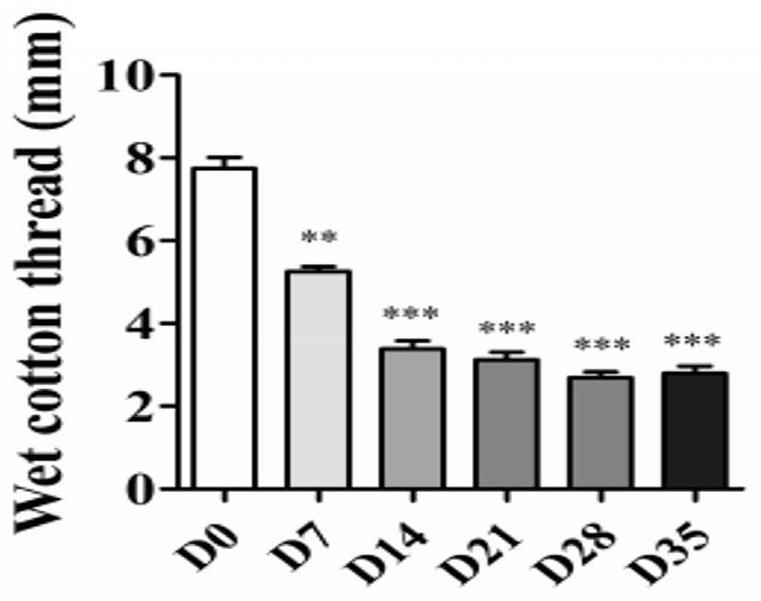
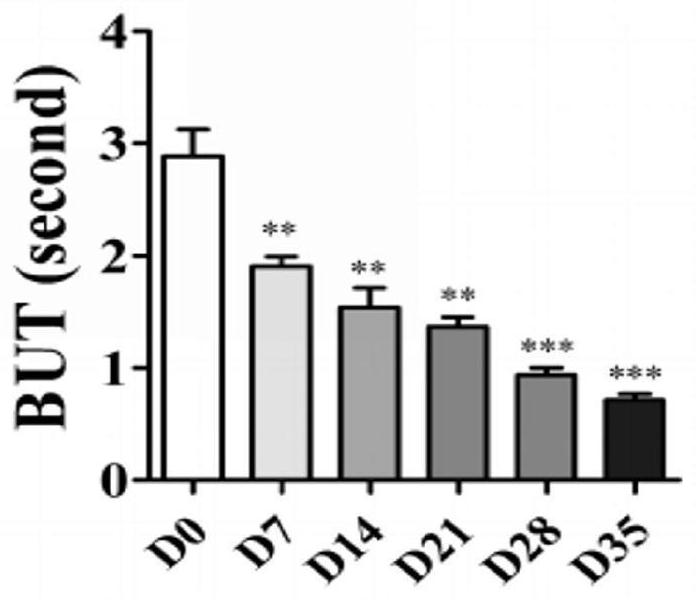
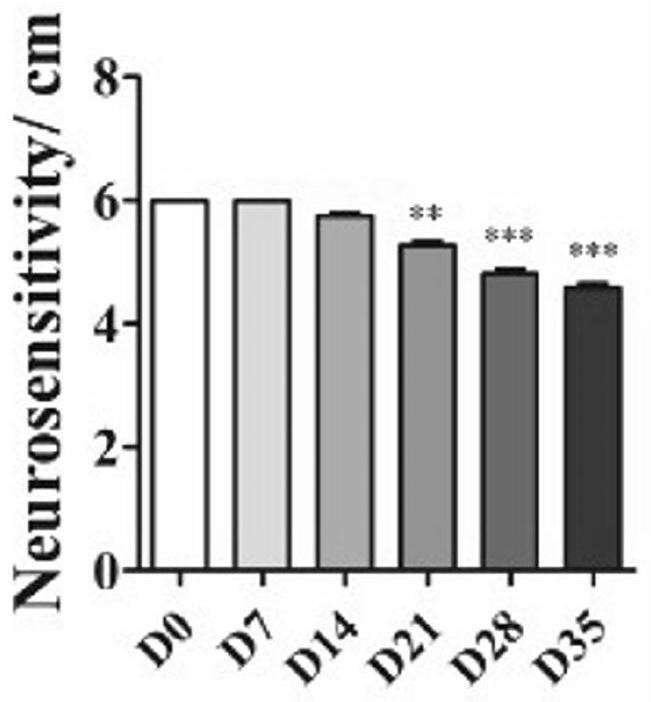
[0066] The basal tear secretion, tear break-up time (BUT) and corneal sensitivity readings of the mice are shown in Figures 1 to 3;
[0068] Mice were harvested on days 7, 14, 21, 28 and 35 of modeling. Performed with an intraperitoneal overdose of 5% chloral hydrate
[0075] 6) Slicing: fix the embedding cassette on the microtome, and slice to near the center of the cornea with a thickness of 5 μm.
[0086] 10) Cover slides: Remove the slides and wipe the xylene from the back and edges of the slides with a paper towel. take advantage of xylene
[0087] The results are shown in Figures 4 to 6. As can be seen from Figure 4, the cornea of the 7D mouse has a slight staining of sodium fluor...
Embodiment 2
[0093]
[0096]
[0097]
[0104] 0.05% sodium fluorescein solution
[0107] 50 μl of goat blocking serum was taken and dissolved in 950 μl of PBS solution, and stored at -20°C until use.
[0110]
[0123] Mice were harvested on days 7 and 14 of treatment. Euthanasia was performed with an intraperitoneal overdose of 5% chloral hydrate, followed by
[0127] The 7D and 14D rhFGF-21 treatment groups, PBS control group and healthy control group mice were treated with eyeballs and
[0129] The HE staining procedure of mouse eyeball and lacrimal gland is as in Example 2.
[0133] 3) 3 times of 0.01M PBS washes, each 5min.
[0135] 5) 3 times of 0.01M PBS washes, each 5min.
[0137] 7) After removing the blocking solution with a paper towel, drop the primary antibody diluted with 1% goat blocking serum, and incubate at 4°C overnight.
[0138] 8) The next day, take out the wet box and rewarm at room temperature for 1 hour.
[0139] 9) 3 times of 0.01M PBS washes, each 5min.
[0141] 11) I...
Embodiment 4
[0152]
[0153]
[0156]
[0157]
[0165] The bagged phosphate buffered saline powder was dissolved in 2L of purified water and mixed thoroughly to obtain 0.01M PBS buffer.
[0168] Add 10 g of paraformaldehyde powder to the PBS solution, dilute to 250 ml, and store in an airtight container. join 1
[0170] 400 mL of methanol was dissolved in 100 mL of purified water to obtain an 80% methanol solution. Take 50mL 30%H
[0177]
[0178]
[0183] Treatment of dry eye mice with combined injection of rhFGF-21 and inhibitor
[0188] The 7D and 14D rhFGF-21 treated, PBS control and healthy control mice were treated with eyeballs and
[0199] (7) 3 times of 0.01M PBS washes, each 5min.
[0201] (9) After removing the blocking solution with a paper towel, drop the primary antibody diluted with 1% goat blocking serum, and incubate at 4°C overnight. and
[0209] (16) 70%, 80%, 90%, and 95% ethanol were dehydrated for 2 min each, and 100% ethanol was dehydrated twice for 1 min each.
[021...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com