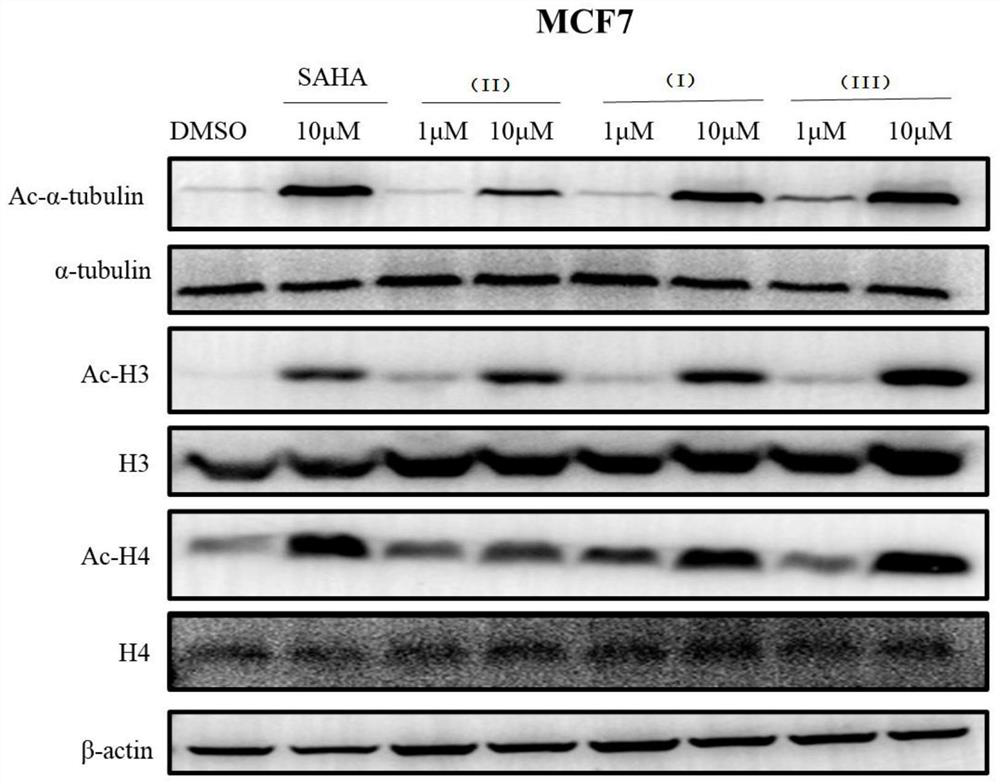
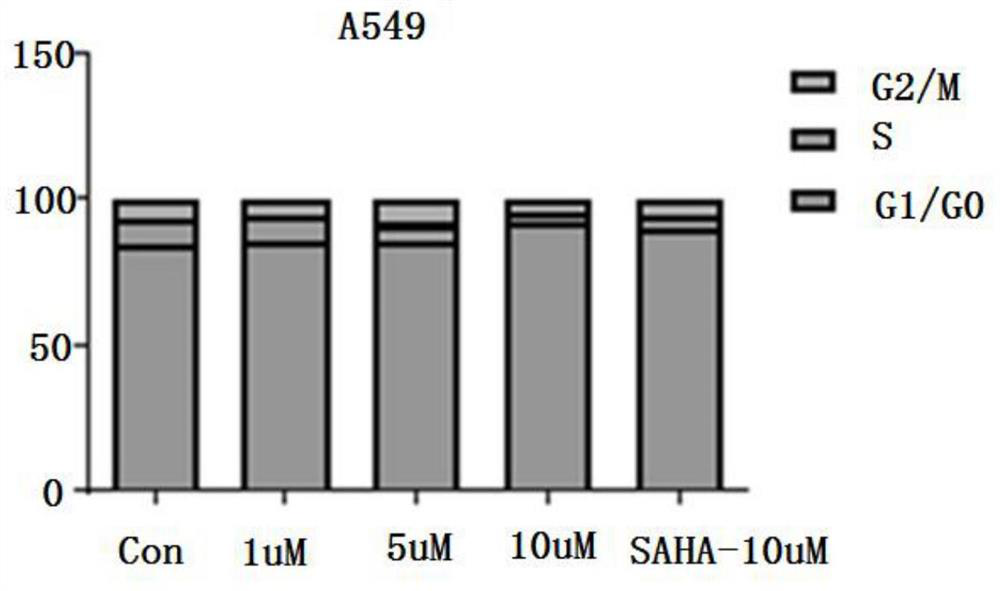
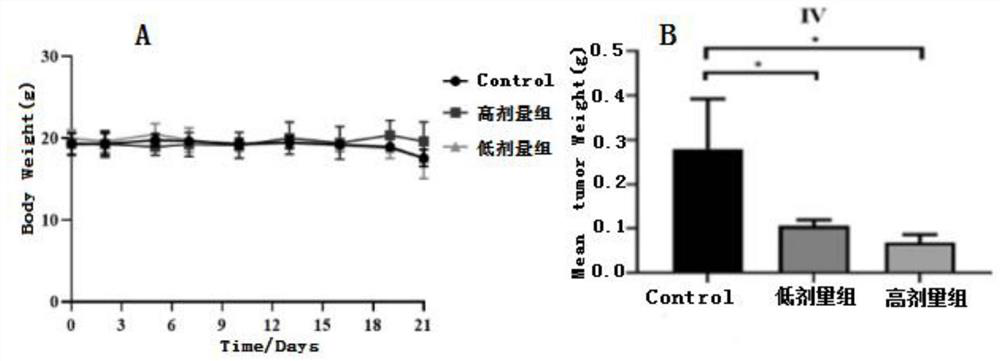
Cysteine derivative, synthesis method and application thereof
A technology of cysteine and synthesis method, which is applied to cysteine derivatives and their synthesis, and the application field of histone deacetylase inhibitors in the preparation of antitumor drugs, which can solve weak therapeutic effects and limit applications. and other problems, to achieve the obvious effect of anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The concrete synthetic method of formula (I) compound, comprises the following steps:
[0032] S1. Take N-((benzyloxy)carbonyl)-S-(4-(tert-butoxy)-4-oxobutyl)-L-cysteine and dry THF (tetrahydrofuran) after stirring and dissolving Add DIEA (N,N-diisopropylethylamine), stir for 5-8 minutes, add HBTU (O-benzotriazole-tetramethyluronium hexafluorophosphate), add aniline after 15-20 minutes, and react at room temperature 3-3.5h; the obtained product was evaporated to remove the solvent under reduced pressure, added dichloromethane to dissolve, then washed the organic layer with HCl solution and saturated NaCl solution, collected the organic layer, dried over anhydrous Na2SO4, concentrated the organic layer, and finally passed through a silica gel column Purified by chromatography to obtain a light yellow solid;
[0033] Wherein, the molar ratio of each raw material to the catalyst is N-((benzyloxy)carbonyl)-S-(4-(tert-butoxy)-4-oxobutyl)-L-cysteine: DIEA: HBTU:aniline=1:...
Embodiment 2
[0048] The specific synthetic method of the compound of formula (I) differs from Example 1 in that in S1, the N-((benzyloxy)carbonyl)-S-(4-(tert-butoxy)-4-oxobutane base)-L-cysteine, the N,N-diisopropylethylamine, the O-benzotriazole-tetramethyluronium hexafluorophosphate and the molar ratio of the aniline is 1 :4:1.5:2.
Embodiment 3
[0050] The specific synthetic method of the compound of formula (I) differs from Example 1 in that in S1, the N-((benzyloxy)carbonyl)-S-(4-(tert-butoxy)-4-oxobutane base)-L-cysteine, the N,N-diisopropylethylamine, the O-benzotriazole-tetramethyluronium hexafluorophosphate and the molar ratio of the aniline is 1 :4:3:2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com