1, 3, 4-oxadiazole derivative as well as preparation method and application thereof
A technology of oxadiazoles and derivatives, applied in different preparation fields, can solve problems such as low solubility, adverse side reactions, low solubility limitation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
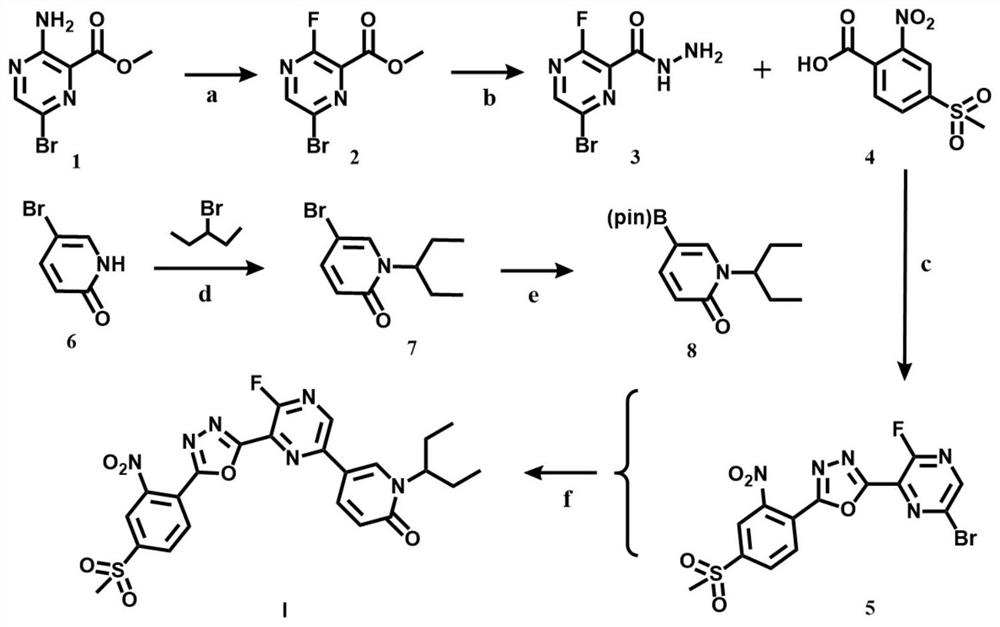
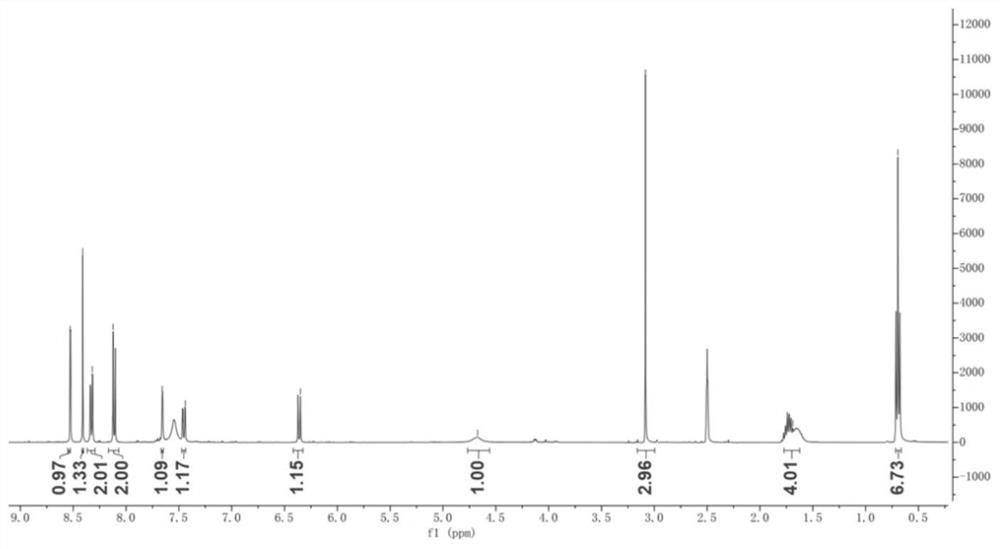
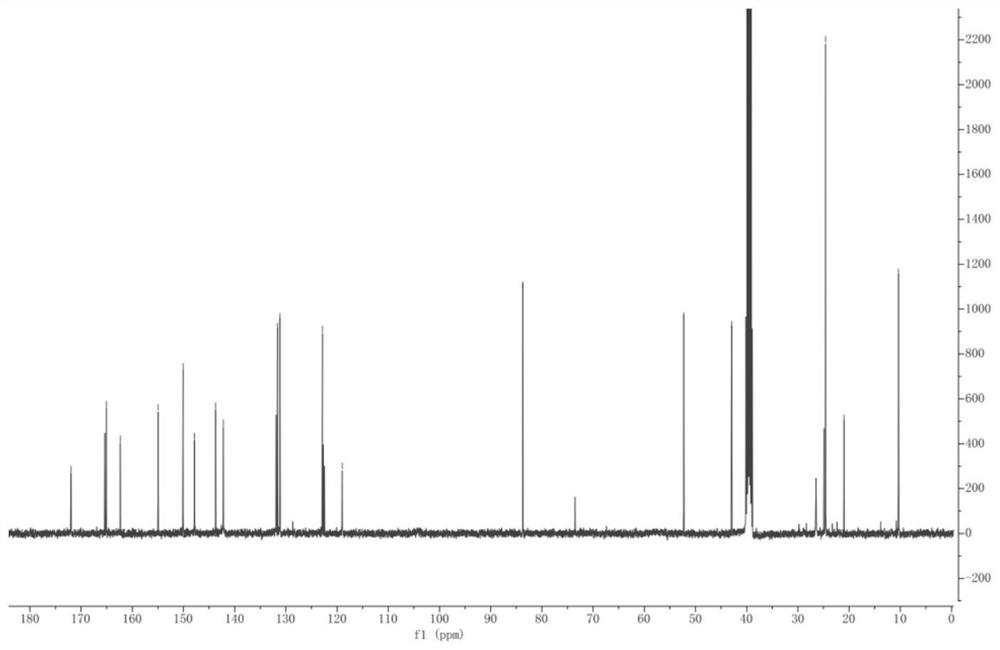
[0023] The present invention will be further described through specific embodiments below in conjunction with the accompanying drawings. These embodiments are only used to illustrate the present invention, and are not intended to limit the protection scope of the present invention.
[0024] The specific implementation method of compound I preparation is as follows:
[0025] Step a: Dissolve methyl 3-amino-6-bromopyrazine-2-carboxylate (5.0 g, 21.55 mmol) in an aqueous solution of hydrochloric acid (5.47 mL, 53.88 mmol) (the volume ratio of hydrochloric acid to water is 1:1) Stir until it becomes a paste, and react at room temperature for 20 minutes. After cooling the reaction system to 0 °C, 20 mL NaNO was added dropwise 2 (1.64g, 23.7mmol) aqueous solution, after fully stirring and reacting at 0°C for 10min, add dropwise HBF with a volume ratio of 40% 4Aqueous solution (14.2 mL, 66.45 mmol), after the dropwise addition was completed, the reaction was continued at room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com