Construction method of GS gene-knockout CHO-K1 cell strain and suspension cell monocloning
A CHO-K1, gene knockout technology, applied in the biological field, can solve the problems of genome contamination, heavy workload, time-consuming and energy-consuming efficiency, etc., to improve the efficiency of GS gene knockout, reduce the risk of contamination, and avoid genome The effect of restructuring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
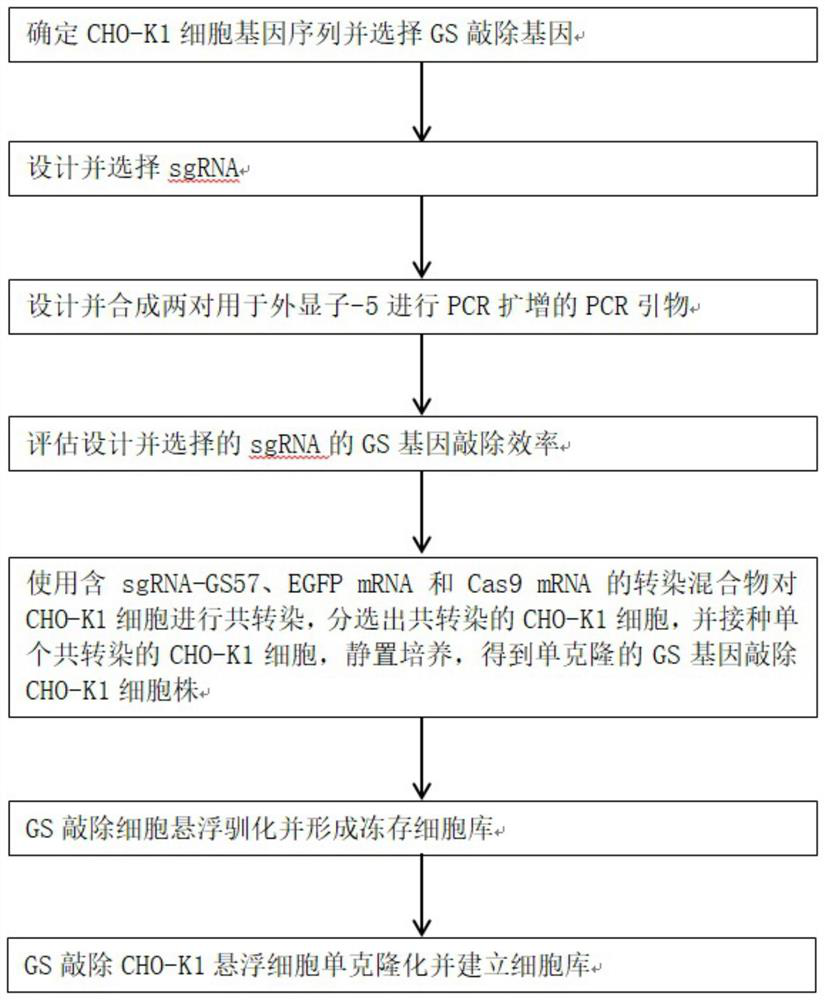
[0044] figure 1 A method for constructing a GS gene knockout CHO-K1 cell line and a method for constructing a suspension cell monoclonal cell bank are shown.
[0045] A method for constructing a GS gene knockout CHO-K1 cell line, comprising the steps of:
[0046] Step A: Based on the nucleotide sequence of GS exon-5 in CHO-K1 cells, design and select sgRNA-GS57 for knocking out the GS gene. The specific operations are as follows:
[0047]Step A1: Determine the gene sequence of CHO-K1 cells and select the GS knockout gene, including the following steps:
[0048] Step A11 Adhesive culture of the CHO-K1 cell line purchased from the American Type Culture Collection (ATCC), the purchased batch number is 62960170;
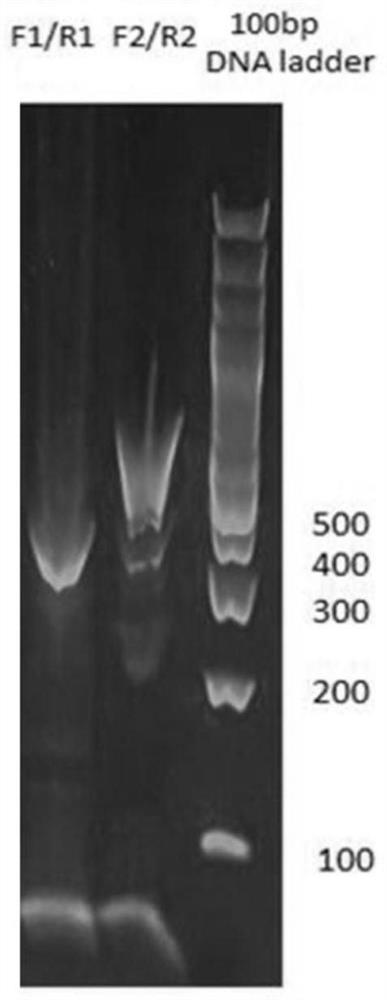
[0049] In the above step A11, the culture conditions are as follows: the culture bottle is selected as T75 culture bottle, the culture medium is selected as F12K medium containing 10% fetal bovine serum, and the amount of culture medium is 15ml; the culture time is 2 d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com