Preparation method of fumaric acid and preparation method of amino acid
A technology of fumaric acid and furoic acid, applied in chemical instruments and methods, fermentation, physical/chemical process catalysts, etc., to achieve mild conditions, realize repeated utilization, and overcome unfriendly effects on the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Resin pretreatment method: soak about 10g macroporous weakly basic styrene-based anion resin (DVB), D201 macroporous strongly basic styrene-based anion-exchange resin, 717 strongly basic styrene-based anion-exchange resin in acetonitrile (50mL) for 30 minutes, filtered and then immersed in methanol (20mL) for 30 minutes and filtered. The separated resin was dried with a rotary evaporator at 50°C until it was free flowing, and then dried in a hot air oven at 50°C for 1 hour.
[0040] The preparation method of immobilized EY: 1 g of macroporous weakly basic styrene-based anion exchange resin (DVB), D201 macroporous strongly basic styrene-based anion-exchange resin, 717 strong-based styrene-based anion-exchange resin, 300 mg of EY Red Y (eosinY, EY) was added into 40mL water and mixed evenly, and adsorbed at 30°C and 100rpm for 10h. The catalyst was filtered, and the filtrate was collected in a 250mL standard flask. The obtained catalyst was ultrasonically cleaned in 10 ...
Embodiment 2
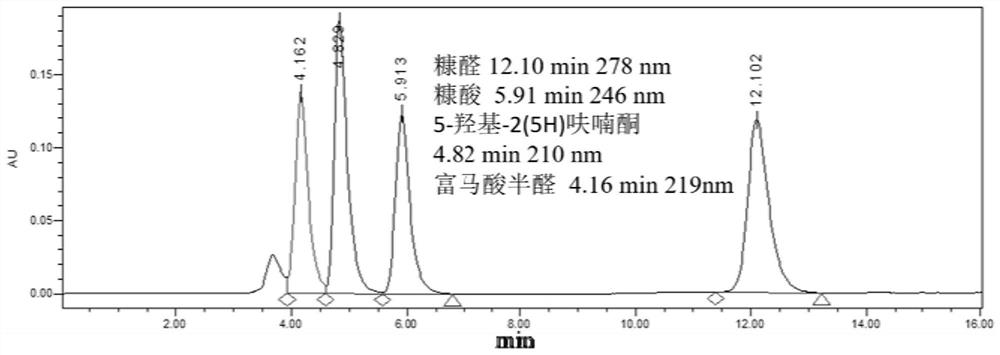
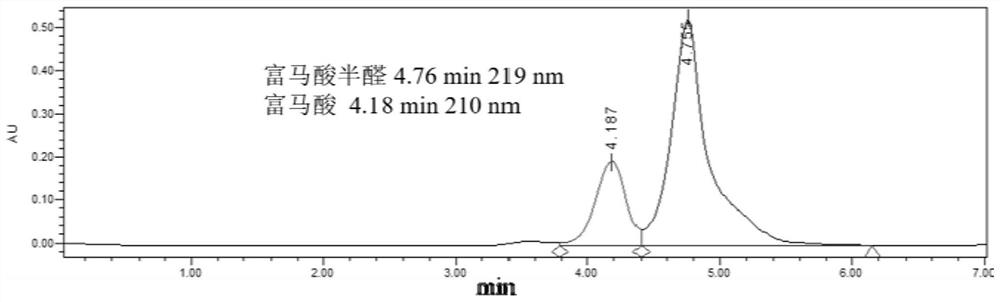
[0042] In 4mL phosphate buffer (200mM, pH 7.0), add 50mg / mL recombinant bacteria E.coli-CtVDH2-NOX (wet weight) and furfural (final concentration 50mM), place in a constant temperature shaker (30°C, 150rpm) React for 2 hours, remove the cells (8000rpm, 4°C, centrifuge for 5min), adjust the pH to 9.0, add the dye EY, place it under 30W 530nm-540nm green light (LED light) to react, the reaction temperature is 25°C, 200rpm, after 24h of reaction , the yield of fumaric semialdehyde (Fumaric semialdehyde, FSA) was 39%.
Embodiment 3
[0044] In 4mL phosphate buffer (200mM, pH 7.0), add 50mg / mL recombinant bacteria E.coli-CtVDH2-NOX (wet weight), and furfural (final concentration 50mM), place in a constant temperature shaker (30°C, 150rpm) React in medium for 2 hours, remove the cells, add the macroporous weakly basic styrene-based anion resin (DVB) prepared in Example 1 to immobilize EY (EY@DVB), the amount of EY@DVB added is 2mol% (final concentration 1mM), Place it under 30W 530nm-540nm green light (LED lamp) for reaction, the reaction temperature is 25°C, 200rpm, after 24 hours of reaction, the yield of fumaric acid semialdehyde is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com