A method of manufacturing a pharmaceutical composition comprising nefopam and acetaminophen, and the pharmaceutical composition obtained thereby
A technique for acetaminophen and its composition, applied in the field of preparing a pharmaceutical composition comprising Nefopam and acetaminophen and the pharmaceutical composition obtained therefrom, capable of solving problems affecting the efficacy and safety of the pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0065] Effect of Process Steps on the Stability of Nefopam
[0066] In order to assess whether the stability of Nefopam is affected by the process step of adding it to the pharmaceutical composition, the following experiments were carried out.
[0067] The method according to the invention involves the preparation of two phases: a granulated powder containing the majority of the components (first process step) and an external phase containing one or more lubricants (second process step).
[0068] Nefopam is added in the granulated powder (first process step) or in the external phase (second process step).
[0069] Two batches using the external phase process and two batches using the internal process were prepared as described below.
[0070] In the context of the present invention, the term % refers to percentage by weight of the composition.
Embodiment I
[0072] Batch F192H043 - Nefopam added in first process step
[0073] Step a): First process step
[0074] In a first process step, a granulated powder was prepared by adding the following components to a planetary mixer: acetaminophen, microcrystalline cellulose (diluent), starch and povidone K90 (binder), cross-linked Sodium carboxymethylcellulose (disintegrant) and anhydrous citric acid (pH adjuster).
[0075] The components were added to the planetary mixer in the following order: first half of acetaminophen, microcrystalline cellulose, starch, PVP K90, Nefopam HCL, croscarmellose sodium, anhydrous citric acid , and finally the second half of acetaminophen.
[0076] 800 g of the powder were mixed for 10 min at 105 rpm.
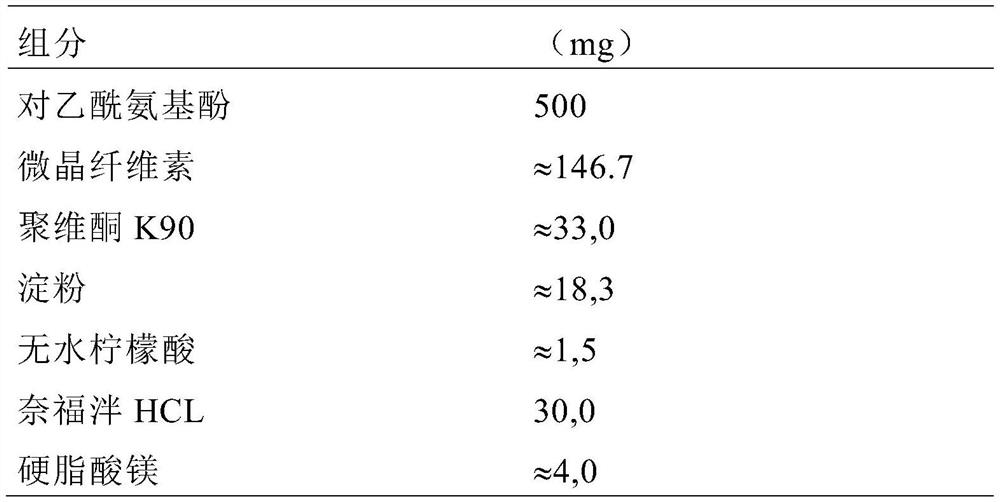
[0077] The final composition of the initial mixture was as follows:
[0078]
[0079] Pure water (235g) was added to the planetary mixer containing the initial mixture, stirred at a constant speed of 80rpm to 115rpm for 20min, and then stirred at 11...
Embodiment II
[0110] Batch F193H044 - Nefopam added in first process step
[0111] first process step
[0112] A granulated powder was prepared as described in Example I for batch F192H043.
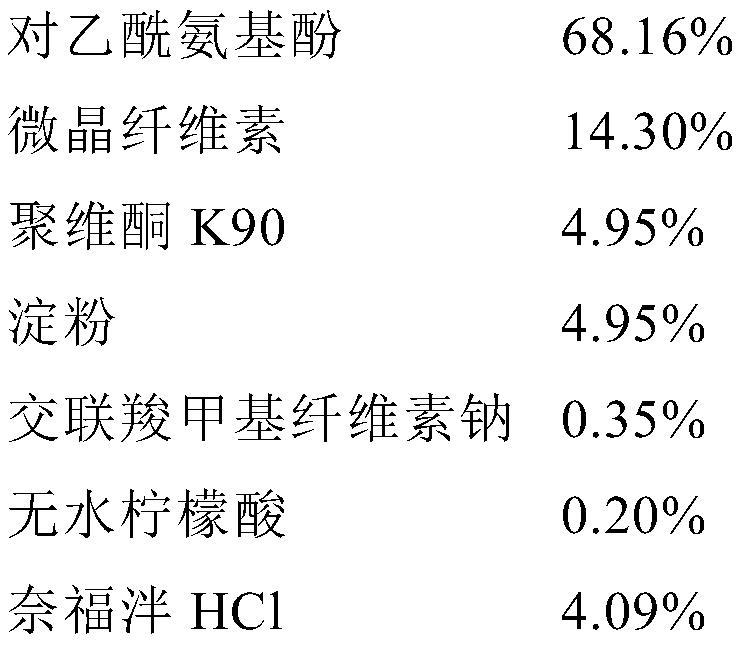
[0113] The final composition of the powder is as follows:
[0114]
[0115] The remaining steps such as drying and quantification were the same as disclosed for batch F192H043.
[0116] second process step
[0117] The second process step was carried out as described in Example 1, the composition of the external phase being:
[0118] Microcrystalline Cellulose 2.02%
[0119] The resulting pharmaceutical composition (357 g) was then tableted.
[0120] Batch F195H046 - Nefopam added in second process step
[0121] first process step
[0122] A granulated powder was prepared as described in Example I.
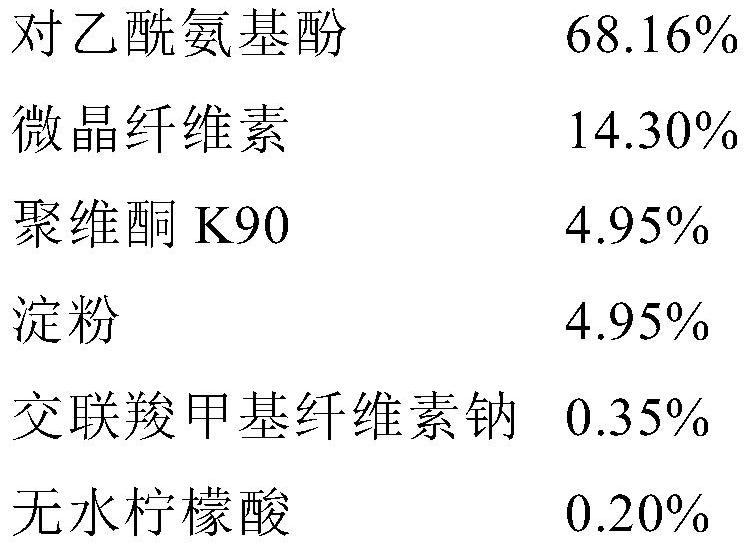
[0123] The final composition of the powder is as follows:
[0124]
[0125] The remaining steps such as drying and quantification were the same as disclosed for batch F194H045.
[0126] s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com