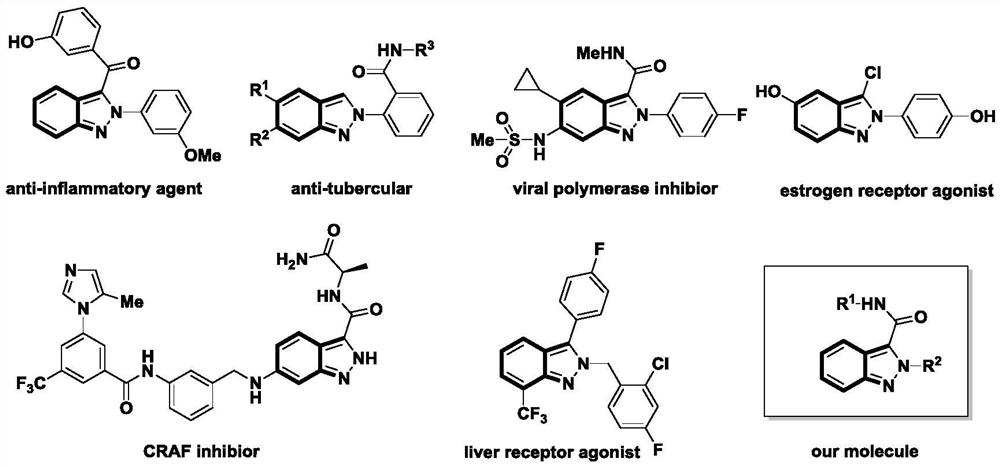
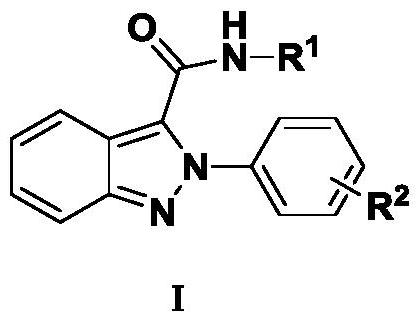
Indazole derivatives and their applications
A technology for indazoles and derivatives is applied in the field of light-induced organic azide preparation of indazole derivatives, and achieves the effects of high yield, mild conditions and good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
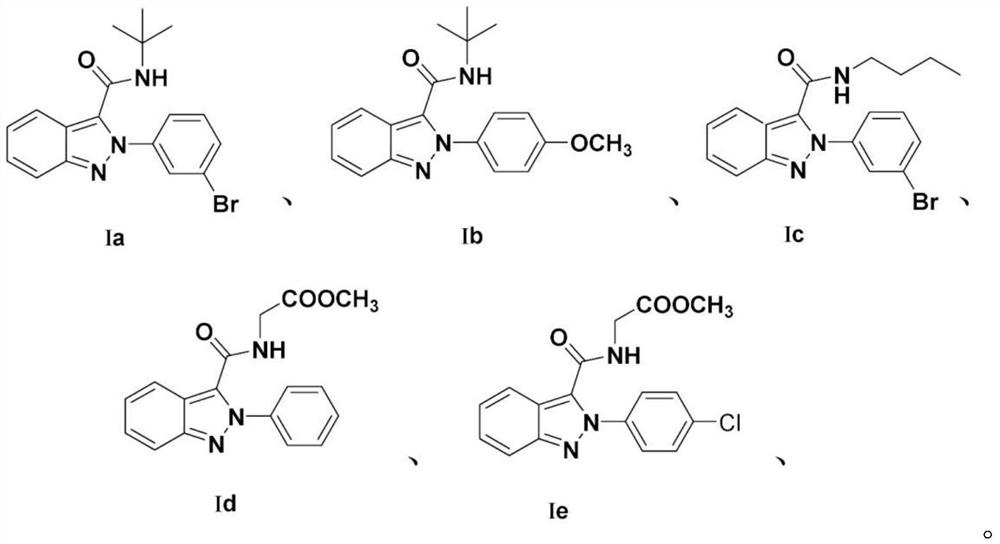
[0043] A method for preparing 2-(3-bromophenyl)-N-(tert-butyl)-2H-indazole-3-carboxamide, comprising the following experimental steps:
[0044] Weigh o-nitrobenzaldehyde (30mmol) into a 100mL round-bottomed flask, add HMPA solvent to fully dissolve, then add sodium azide (60mmol) to dissolve, and place it in a constant temperature oil bath at 60°C to start the reaction. After the reaction was monitored by TLC, the reaction solution was left to cool to normal temperature, and poured into an ice-water solution under uniform stirring and left to stand. It was observed that a light yellow solid was gradually precipitated. The post-treatment was simply filtered, washed and dried to obtain adjacent alkene Nitrobenzaldehyde. Take o-azidobenzaldehyde (1mmol, 1.0eqv.) and dissolve it in 2mL of methanol solvent, add equimolar 3-bromoaniline (1mmol, 1.0eqv.) and 0.2mmol of H 3 PO 4 After the reaction was carried out for 5 min, 1 mL of methanol was added for a complete reaction, and the...
Embodiment 2
[0048] A method for preparing N-(tert-butyl)-2-(4-methoxyphenyl)-2H-indazole-3-carboxamide, comprising the following experimental steps:
[0049] Weigh o-nitrobenzaldehyde (30mmol) into a 100mL round-bottomed flask, add HMPA solvent to fully dissolve, then add sodium azide (60mmol) to dissolve, and place it in a constant temperature oil bath at 60°C to start the reaction. After the reaction was monitored by TLC, the reaction solution was left to cool to normal temperature, and poured into an ice-water solution under uniform stirring and left to stand. It was observed that a light yellow solid was gradually precipitated. The post-treatment was simply filtered, washed and dried to obtain adjacent alkene Nitrobenzaldehyde. Take azidobenzaldehyde (1mmol, 1.0eqv.) and dissolve it in 2mL of methanol solvent, add equimolar 4-methoxyaniline (1mmol, 1.0eqv.) and 0.2mmol of H 3 PO 4 After the reaction was carried out for 5 min, 1 mL of methanol was added for a complete reaction, and t...
Embodiment 3
[0053] A method for preparing 2-(3-bromophenyl)-n-butyl-2H-indazole-3-carboxamide, comprising the following experimental steps:
[0054] Weigh o-nitrobenzaldehyde (30mmol) into a 100mL round-bottomed flask, add HMPA solvent to fully dissolve, then add sodium azide (60mmol) to dissolve, and place it in a constant temperature oil bath at 60°C to start the reaction. After the reaction was monitored by TLC, the reaction solution was left to cool to normal temperature, and poured into an ice-water solution under uniform stirring and left to stand. It was observed that a light yellow solid was gradually precipitated. The post-treatment was simply filtered, washed and dried to obtain adjacent alkene Nitrobenzaldehyde. Take o-azidebenzaldehyde (1mmol, 1.0eqv.) and dissolve it in 2mL of methanol solvent, add equimolar 4-methoxyaniline (1mmol, 1.0eqv) and 0.2mmol of H 3 PO 4 After the reaction was carried out for 5 minutes, 1 mL of methanol was added for a complete reaction, and then,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com