Aie compounds with fluorescence, photoacoustic, and raman properties
A compound and conjugation technology, applied in organic chemistry, color-changing fluorescent materials, luminescent materials, etc., can solve problems such as difficult to propose molecular criteria and difficult to obtain organic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
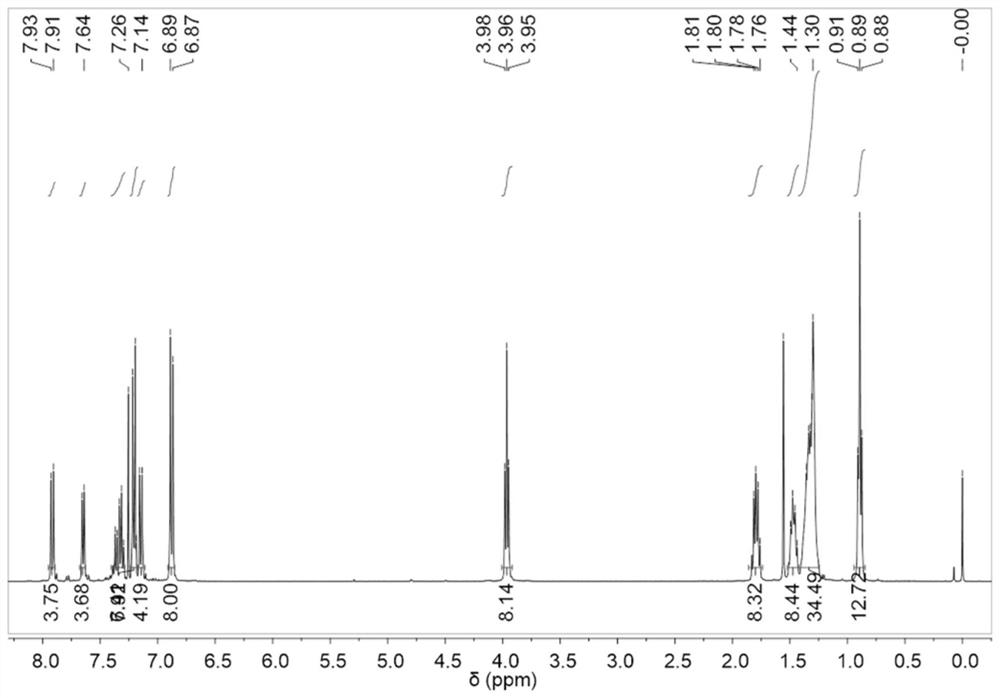
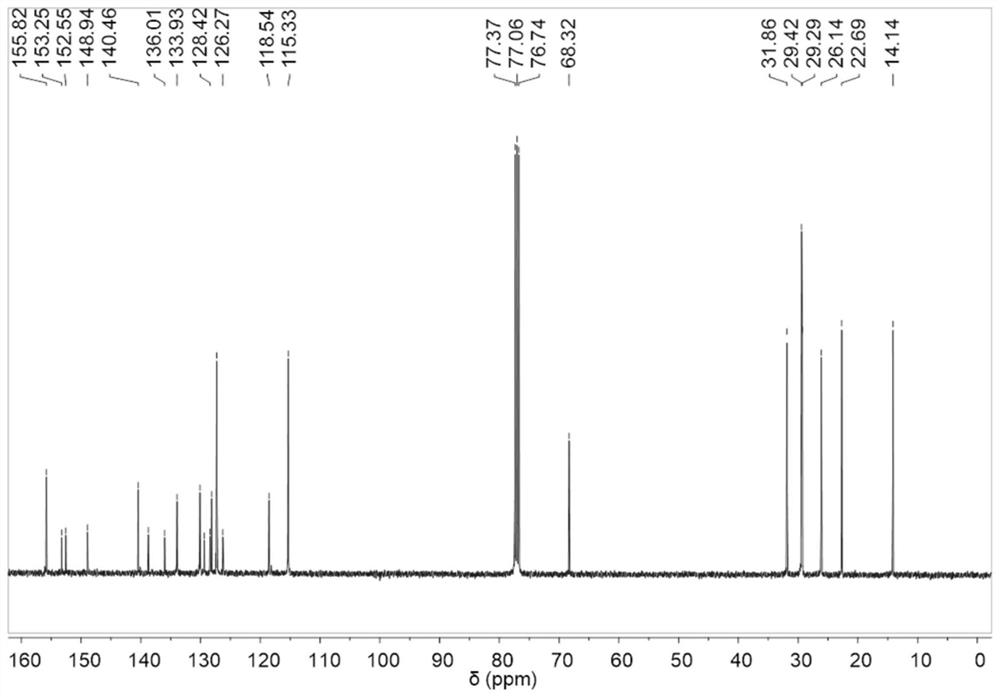
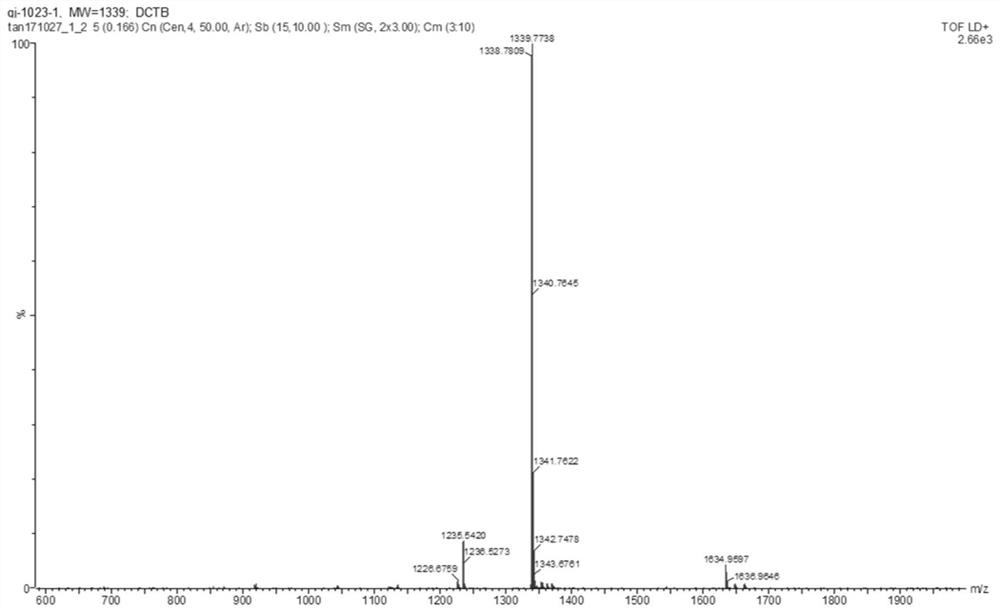
[0241] Synthesis and characterization of OTPA-TQ
[0242] Synthesis of 1-iodo-4-(octyloxy)benzene (2): 1-bromooctane (4.24g, 22mmol), 4-iodophenol (4.4g, 20mmol) and K 2 CO 3 (8.3 g, 60 mmol) was added to a 250 mL two necked round bottom flask and the flask was evacuated and purged 3 times with dry nitrogen. Anhydrous DMF (120 mL) was then added, and the mixture was heated to reflux and stirred for 24 hours. After cooling to room temperature, water was added, and the mixture was washed 3 times with dichloromethane. Combine the organic phases with MgSO 4 Dry, and evaporate the solvent under reduced pressure. The crude product was purified by column chromatography on silica gel using dichloromethane / hexane (v / v 1:10) as eluent to afford 1-iodo-4-(octyloxy)benzene as a colorless oil (yield 83%). 1 H NMR (400MHz, CDCl 3 ):δ7.53(d,2H),6.86(d,2H),3.90(t,2H),1.82-1.70(m,2H),1.48-1.39(m,2H),1.39-1.21(m,8H ),0.89(t,3H). 13 CNMR (100MHz, CDCl 3 ): δ159.02, 138.14, 116.94, 82...
Embodiment 2
[0244] Synthesis of 4-bromo-N,N-bis(4-(octyloxy)phenyl)aniline (4)
[0245] 4-bromoaniline (1.03g, 6mmol), 1-iodo-4-(octyloxy)benzene (4.98g, 15mmol), 1,10-phenanthroline (0.18g, 1mmol), CuI (2.12g, 0.19 mmol) and KOH (5.04 g, 90 mmol) were added to a 250 mL two necked round bottom flask. Dry toluene (50 mL) was added to the flask under nitrogen atmosphere. The flask was then evacuated and purged three times with dry nitrogen, and the mixture was heated to reflux and stirred for 24 hours. After cooling to room temperature, water was added, and the mixture was washed 3 times with dichloromethane. Combine the organic phases with MgSO 4 Dry, and evaporate the solvent under reduced pressure. The crude product was purified by column chromatography on silica gel using dichloromethane / hexane (v / v 1:6) as eluent to afford 4-bromo-N,N-bis(4-(octyloxy) as a viscous oil ) phenyl) aniline (yield 76%). 1 H NMR (400MHz, CDCl 3 ):δ7.22(d,2H),7.00(d,4H),6.79(t,6H),3.92(t,4H),1.81-1.7...
Embodiment 3
[0247] Synthesis of 4-(octyloxy)-N-(4-(octyloxy)phenyl)-N-(4-(tributylstannyl)phenyl)aniline (5)
[0248] 4-Bromo-N,N-bis(4-(octyloxy)phenyl)aniline (2.32 g, 4 mmol) was added to a 100 mL two necked round bottom flask. The flask was then evacuated and flushed three times with dry nitrogen, and anhydrous THF (50 mL) was added. The mixture was then cooled to −78° C. with a dry ice-acetone mixture and kept at this temperature for 15 minutes before adding n-butyllithium ( n BuLi, 2.5M in hexane, 1.6 mL, 4 mmol). After stirring at this temperature for 2 hours, tri-n-butyltin chloride (1.1 mL, 4 mmol) was added, and the mixture was slowly warmed to room temperature and stirred overnight. After that, water was added to quench the reaction, and the mixture was extracted three times with dichloromethane. Combine the organic phases with MgSO 4 Dry, and evaporate the solvent under reduced pressure. The crude product was used without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com