Bonded polyurethane nonlinear optical material as well as preparation method and application thereof
A technology of nonlinear optics and polyurethane, applied in the field of optical materials, can solve the problem of few reports on optical nonlinearity of composite materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A kind of preparation of bonding type polyurethane S1 / PU nonlinear optical material, comprises the following steps:
[0052]a. Add 0.115 g (0.7 mmol) of azobisisobutyronitrile (AIBN) to 7.8 g (0.06 mol) of hydroxyethyl methacrylate (HEMA), stir to dissolve it completely, and obtain a HEMA solution in which AIBN is dissolved.
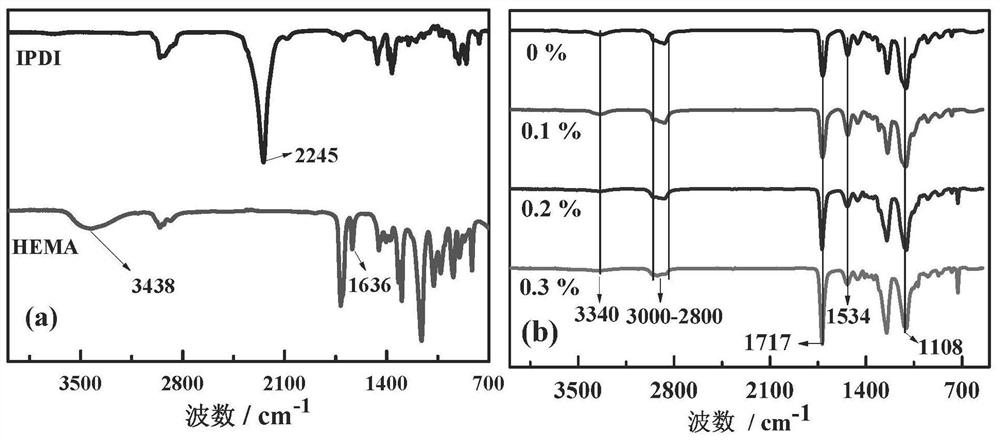
[0053] b. In a 125mL round bottom flask, add 39mg (0.08mmol) of compound S1 to 18g (0.03mol) of polyethylene glycol (PEG600), and add 13.36g (0.06mol) of isophorone diisocyanate (IPDI ). Stir mechanically for 5 to 10 minutes at a water bath temperature of 25° C., and then add 0.037 g of dibutyltin dilaurate (DBTL) as a catalyst. After the reaction solution becomes clear, keep stirring for 15-20 minutes, then add the HEMA solution of AIBN, react for 15-20 minutes, and then stop stirring. Polyethylene glycol (PEG600), compound S1, hydroxyl groups on HEMA were reacted with isocyanate groups on IPDI.
[0054] c. Then use a vacuum pump to remove the...
Embodiment 2
[0062] A kind of preparation of bonding type polyurethane S2 / PU nonlinear optical material, comprises the following steps:
[0063] The preparation process of S2 / PU is the same as the preparation process of S1 / PU nonlinear optical material, only need to replace compound S1 in step b with compound S2, and finally obtain S2 / PU nonlinear optical material.
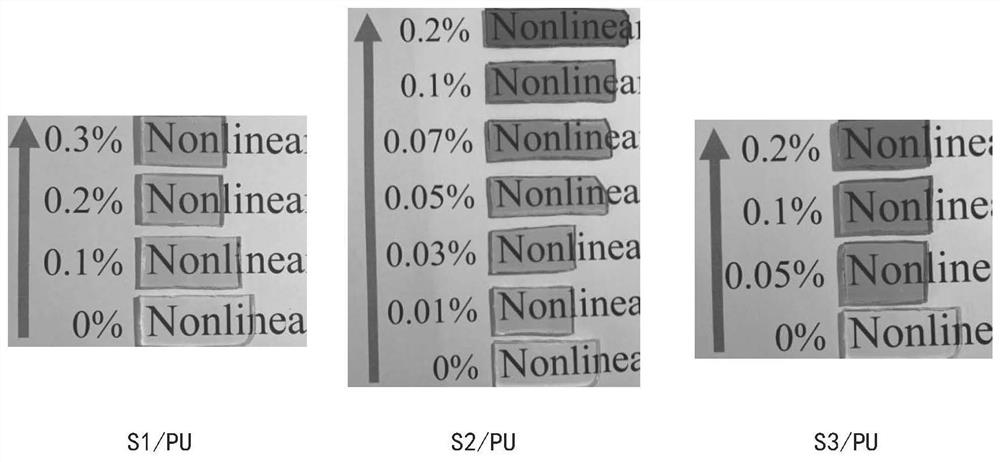
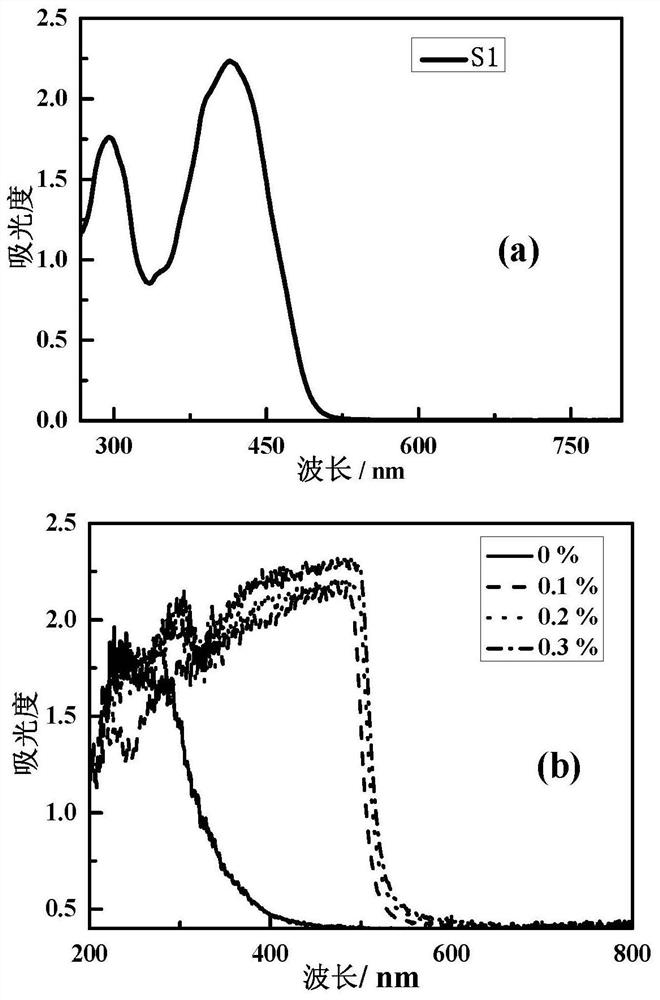
[0064] Adjust the amount of compound S2 to obtain S2 / PU nonlinear optical materials without compound S2 and with a mass content of compound S2 of 0.01%, 0.03%, 0.05%, 0.07%, 0.1%, and 0.2%, respectively. Physical picture such as figure 1 shown.
[0065] Depend on figure 1 It can be seen that with the increase of the compound S2 content, the optical color of the S2 / PU nonlinear optical material gradually changes from yellow to red, and also has good optical transparency, and can be used as an optically transparent material.
Embodiment 3
[0067] A kind of preparation of bonding type polyurethane S3 / PU nonlinear optical material, comprises the following steps:
[0068] The preparation process of the S3 / PU sheet is the same as the preparation process of the S1 / PU nonlinear optical material, only need to replace the compound S1 in step b with the compound S3, and finally obtain the S3 / PU nonlinear optical material.
[0069] Adjust the amount of compound S3 to obtain S3 / PU nonlinear optical materials that do not contain compound S3 and have a mass content of 0.05%, 0.1%, and 0.2% of compound S3 respectively. The physical pictures of each optical material are as follows figure 1 shown.
[0070] Depend on figure 1 It can be seen that with the increase of the content of the compound S3, the color of the S3 / PU nonlinear optical material gradually deepens, has good optical transparency, and can be used as an optically transparent material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com