Primer, probe and kit for detecting gene mutation sites related to drug resistance of mycobacterium tuberculosis and treatment drugs
A technology of Mycobacterium tuberculosis and mutation sites, applied in the field of medical testing, can solve the problems of increasing the risk of PCR contamination and false positives, not covering the detection of pyrazinamide and streptomycin resistance, and long experiment cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0247] The known sample 1 contains isoniazid and rifampicin-resistant Mycobacterium tuberculosis, which is detected by the method of the present invention, and the specific steps are as follows:
[0248] a. Extract the total DNA in the sample;
[0249] b. First use the total DNA of the sample extracted in step a as a template to perform PCR. Add the following substances to the PCR tube: 2×Multiplex PCRMasterMix mixture (including four deoxyribonucleotides, PCR buffer, Taq DNA polymerase) 12.5 μL, IS6110-F, IS6110-R, IS6110-R, 16S-F, 16S-R, katG-F, katG-R, inhA-F, inhA-R, rpoB-F, rpoB-R, gyrA-F, gyrA-R, rpsL-F, rpsL-R, rrs- F, rrs-R, pncA-F and pncA-R each 0.125 μL, DNA template 1 μL and ultrapure water 9.25 μL.
[0250] The sequences of each primer are as follows:
[0251] IS6110-F: biotin-CATGTCCGGAGACTCCAGTT
[0252] IS6110-R: GGTACCTCCTCGATGAACCA
[0253] 16S-F: biotin-TAGATACCCTGGTAGTCC
[0254] 16S-R: CGACACGAGCTGACGACA
[0255] katG-F: biotin-CTCGGCGATGAGCGTTACA ...
Embodiment 2
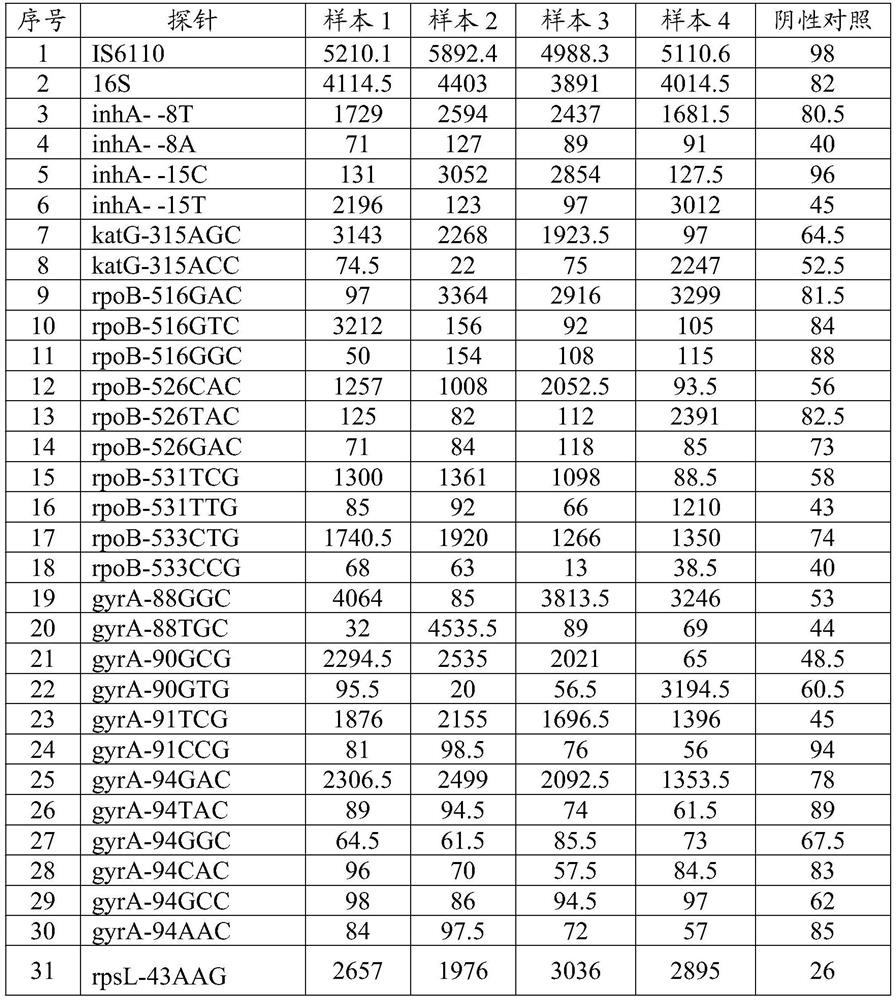
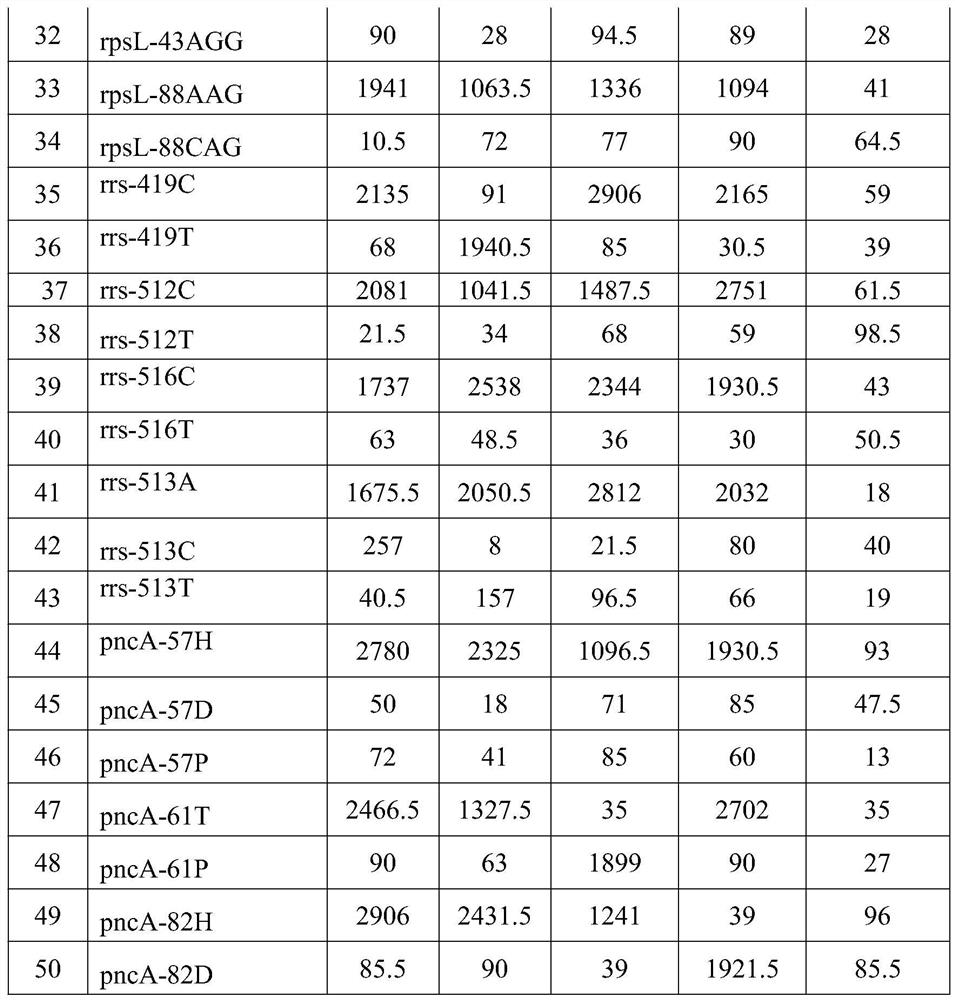
[0335] Known sample 2 contains fonoquinones and streptomycin-resistant Mycobacterium tuberculosis, which is detected by the method of the present invention, and the steps are the same as in Example 1, and the results are as shown in Table 1. The fluorescence value of the probe IS6110 of the mycobacterium-related gene IS6110 is 5892.4, and the fluorescence value of the 16S probe 16S is 4403, which is greater than 100, indicating that it contains Mycobacterium tuberculosis.
[0336] The fluorescence value of the mutant probe katG-315ACC at the 315 amino acid mutation site of the isoniazid resistance-related gene katG gene in this sample is 2268, which is greater than 100, and is more than ten times the fluorescence value of the mutant probe katG-315AGC. Therefore, this sample does not contain drug resistance mutations related to katG-315 amino acid. The fluorescence value of the wild-type probe inhA--15C at the 15th amino acid mutation site of the inhA gene related to isoniazid ...
Embodiment 3
[0343] Known sample 3 contains pyrazinamide drug-resistant Mycobacterium tuberculosis, and is detected by the method of the present invention, and concrete steps are identical with example 1, and the result of fluorescence detection value is as shown in table 1, and this sample Mycobacterium tuberculosis is relevant The fluorescence value of the probe IS6110 of the gene IS6110 is 4988.3, and the fluorescence value of the 16S probe 16S is 3891, which is greater than 100, indicating that it contains Mycobacterium tuberculosis.
[0344] The fluorescence value of the wild-type probe katG-315AGC at amino acid 315 of the isoniazid resistance-related gene katG gene in this sample is 1923.5, which is greater than 100, and is more than ten times the fluorescence value of the mutant probe katG-315ACC, so this sample is not Contains drug resistance mutations related to katG-315 amino acid. The fluorescence value of the wild-type probe inhA--15C at the -15 amino acid mutation site of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com