Establishment method and application of uplc fingerprint of Xintong Granules
A technique of fingerprinting and establishing methods, which is applied in the field of analysis of traditional Chinese medicine preparations, achieves good stability and repeatability, saves time and material costs, and overcomes the effects of single detection indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
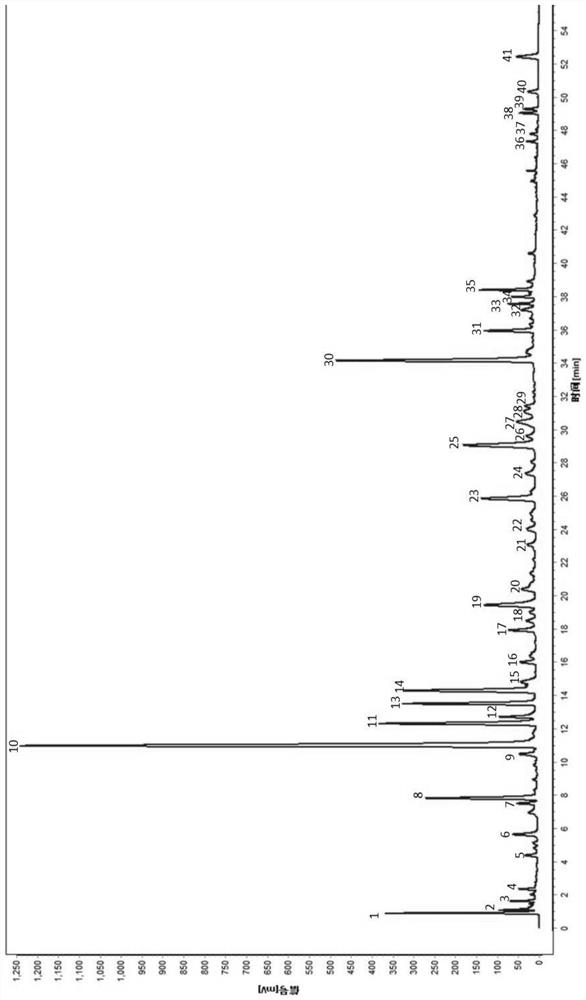
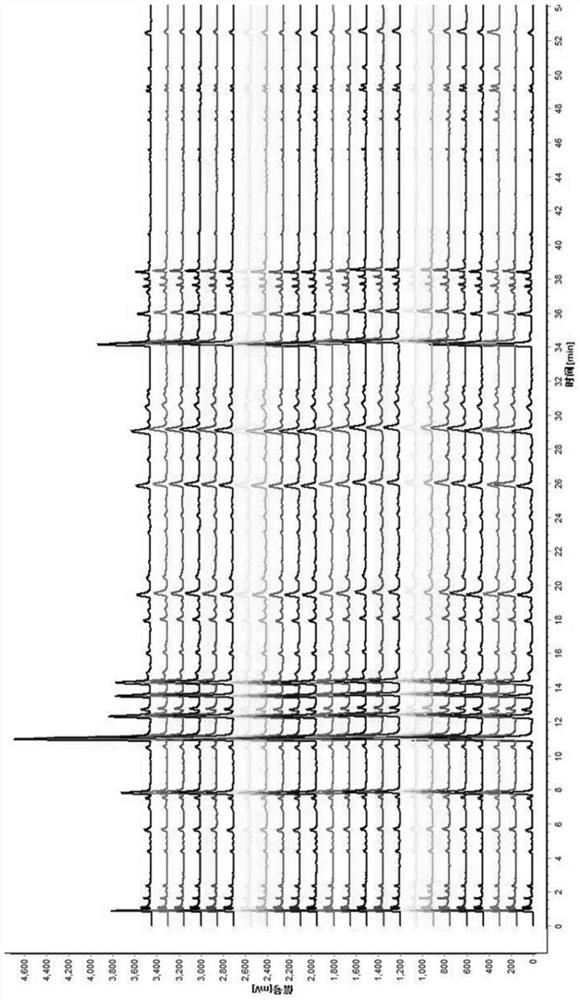
[0049] The establishment of embodiment 1 Xintong granule UPLC standard fingerprint
[0050] 1 Instruments and reagents
[0051] 1.1 Instrument
[0052] Waters Acquity Arc high performance liquid chromatography (USA); 2998PDA detector; Quaternary ultra-high pressure gradient pump; Empower chromatography workstation.
[0053] 1.2 Reagent
[0054] Xintong Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., and the sample batch numbers are shown in Table 1; acetonitrile was chromatographically pure, water was double distilled water, and the rest of the reagents were analytically pure.
[0055] Table 1 Xintong Granule Test Sample Batch Number
[0056]
[0057]
[0058] 2 Methods and results
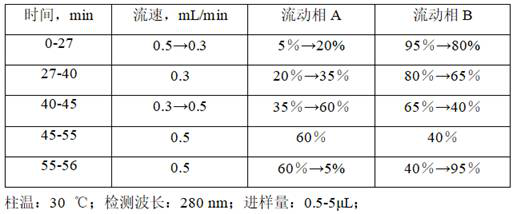
[0059] 2.1 Chromatographic conditions: Chromatographic column: C18 (4.6×50mm, 2.7μm) column; mobile phase: acetonitrile as mobile phase A, 0.1% phosphoric acid aqueous solution as mobile phase B, detection wavelength: 280nm; column temperature: 30°C; injection volume :...
Embodiment 2
[0089] The establishment of embodiment 2 Xintong granules UPLC standard fingerprint
[0090] 1 Instruments and reagents
[0091] 1.1 Instrument
[0092] Waters Acquity Arc high performance liquid chromatography (USA); 2998PDA detector; Quaternary ultra-high pressure gradient pump; Empower chromatography workstation.
[0093] 1.2 Reagent
[0094] Xintong Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., and the sample batch numbers are shown in Table 4. Acetonitrile was chromatographically pure, water was double distilled water, and other reagents were analytically pure.
[0095] Table 4 Xintong Granule Test Sample Batch Number
[0096]
[0097] 2 Methods and results
[0098] 2.1 UPLC chromatographic conditions: Chromatographic column: C18 (4.6×50mm, 2.7μm) column; mobile phase: acetonitrile as mobile phase A, 0.06% phosphoric acid aqueous solution as mobile phase B; detection wavelength: 275nm; column temperature: 28°C; injection volume : 2 μl.
[009...
Embodiment 3
[0105] The establishment of embodiment 3 Xintong granules UPLC standard fingerprint
[0106] 1 Instruments and reagents
[0107] 1.1 Instrument
[0108] Waters Acquity Arc high-performance liquid chromatography (USA): 2998PDA detector; quaternary ultra-high pressure gradient pump; Empower chromatography workstation.
[0109] 1.2 Reagent
[0110] Xintong Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., and the sample batch numbers are shown in Table 5. Acetonitrile was chromatographically pure, water was double distilled water, and other reagents were analytically pure.
[0111] Table 5 Xintong Granule Test Sample Batch Number
[0112]
[0113]
[0114] 2 Methods and results
[0115] 2.1 Chromatographic conditions: Chromatographic column: C18 (4.6×50mm, 2.7μm) column; mobile phase: acetonitrile as mobile phase A, and 0.08% phosphoric acid aqueous solution as mobile phase B; detection wavelength: 277nm; column temperature: 29°C; injection volume : 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com