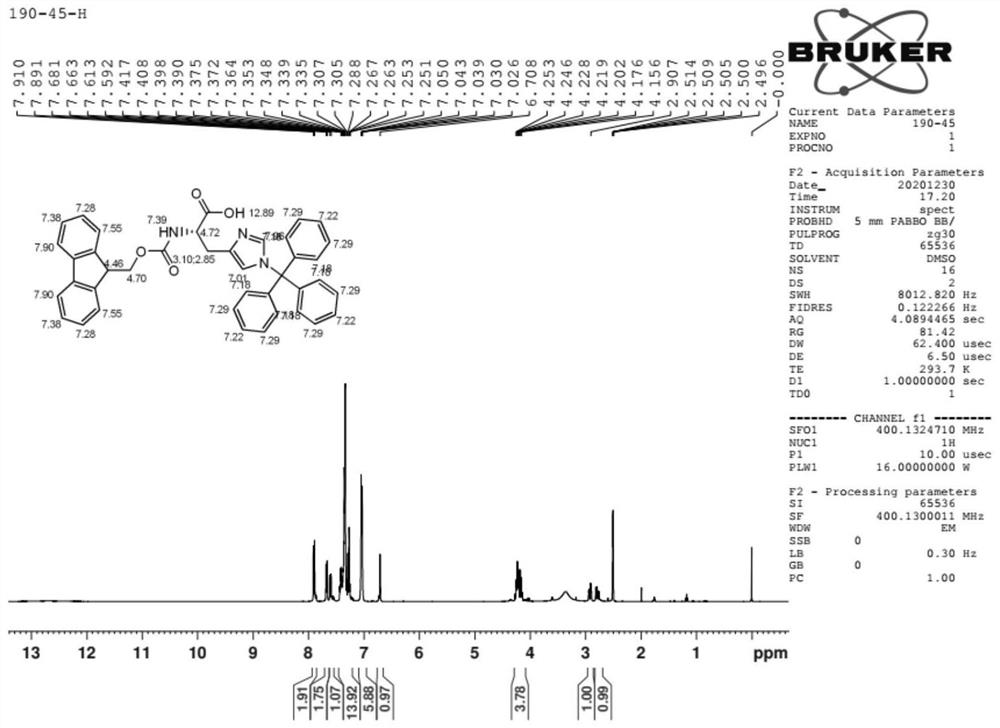
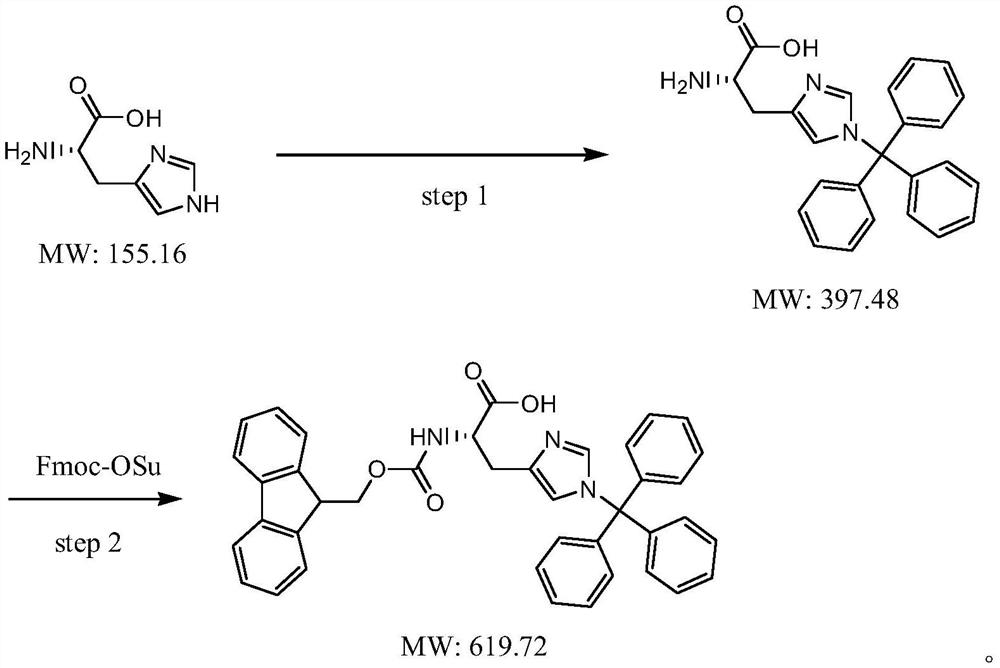
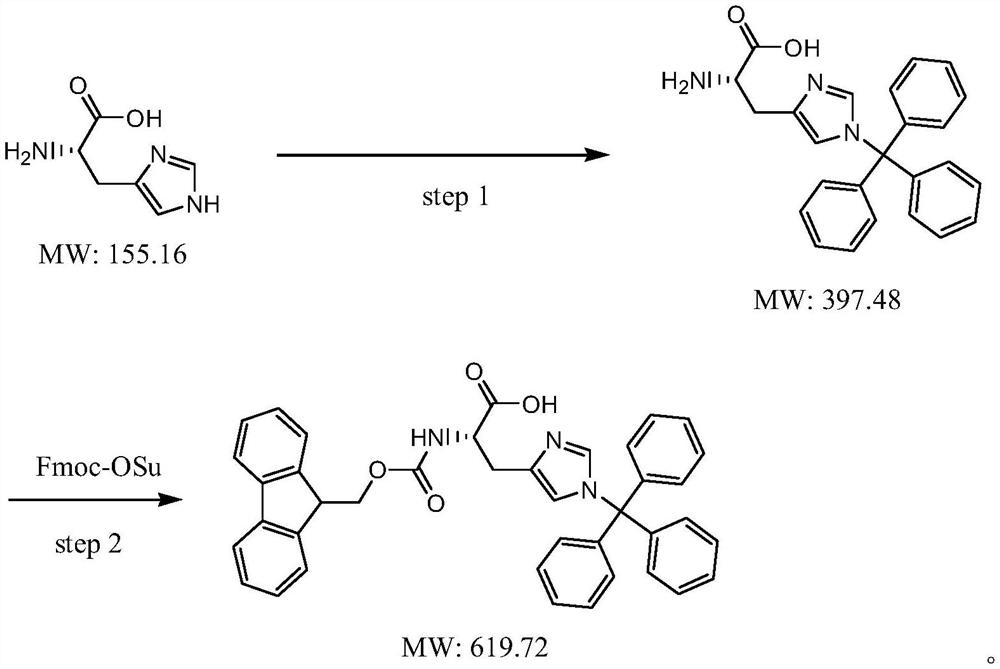
Preparation method of N-9-fluorenyl methoxycarbonyl-N'-trityl-L-histidine
A technology of fluorenyl methoxycarbonyl and trityl histidine, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of lower quality of downstream products, and achieve the effects of simple operation, high yield, and low overall production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 500 g of dichloromethane into a clean 2L four-neck flask, add 50 g of L-histidine (molecular weight 155.2, 0.35 mol) while stirring, and heat up to reflux. 67 g of dichlorodimethylsilane (molecular weight 129.1, 0.52 mol) was added dropwise, and the addition was completed in about 2 hours. After the addition, the reaction was kept under reflux for about 6 hours. After the reaction, add 120g of triethylamine (molecular weight: 101.2, 1.19mol) dropwise, then reflux for about half an hour, cool down to about 15°C, and then add 35g of triethylamine (molecular weight: The mixed solution of 100g of methyl chloride (molecular weight: 278.8, 0.36mol) and 100g of dichloromethane was added dropwise in about 4 hours, and then kept at 15-20°C for about 2 hours. Control until the raw materials disappear, add dropwise a mixed solvent of 50 g of methanol and 100 g of ethyl acetate, and stir for about 2 hours after the addition. Suction filtration, the collected solid was rinsed t...
Embodiment 2
[0033] Add 800 g of dichloromethane into a clean 2L four-neck flask, add 50 g of L-histidine (molecular weight 155.2, 0.35 mol) while stirring, and heat up to reflux. 75 g of dichlorodimethylsilane (molecular weight: 129.1, 0.58 mol) was added dropwise, and the addition was completed in about 2 hours. After the reaction, add 70.8g of triethylamine (molecular weight: 101.2, 0.70mol) dropwise, then reflux for about half an hour, cool down to about 15°C, then add 35g of triethylamine (molecular weight: 101.2, 0.35mol), triphenyl The mixed solution of 117g of methyl chloride (molecular weight: 278.8, 0.42mol) and 100g of dichloromethane was added dropwise in about 4 hours, and the reaction was carried out at 15-20°C for about 2 hours after the addition. Control until the raw materials disappear, add dropwise a mixed solvent of 50 g of methanol and 100 g of ethyl acetate, and stir for about 2 hours after the addition. Suction filtration, collect the solid, rinse twice with about 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com