SiRNA delivery carrier and preparation method and application thereof
A delivery carrier and drug-carrying technology, which is applied in drug delivery, pharmaceutical formulation, liposome delivery, etc., can solve the problems of low yield of natural exosomes, complex components of exosomes, and difficulty in controlling the effect, and achieve low equipment prices , high encapsulation efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Another aspect of the embodiments of the present invention also provides a method for preparing the aforementioned siRNA delivery carrier, which includes:
[0047] Preparation of red blood cell membranes into nanovesicles;
[0048] siRNA and cationic liposomes are incubated to prepare siRNA / cationic liposome complexes;
[0049] And, the siRNA / cationic liposome complex is loaded in the nanovesicle to obtain the siRNA delivery carrier.
[0050] In some more specific embodiments, the preparation method includes: co-incubating siRNA and cationic liposomes for 20-30 min to prepare the siRNA / cationic liposome complex.
[0051] In some more specific embodiments, the preparation method includes: ultrasonically treating the siRNA / cationic liposome complex and nanovesicles for 1.0-15 min to obtain the siRNA delivery carrier.
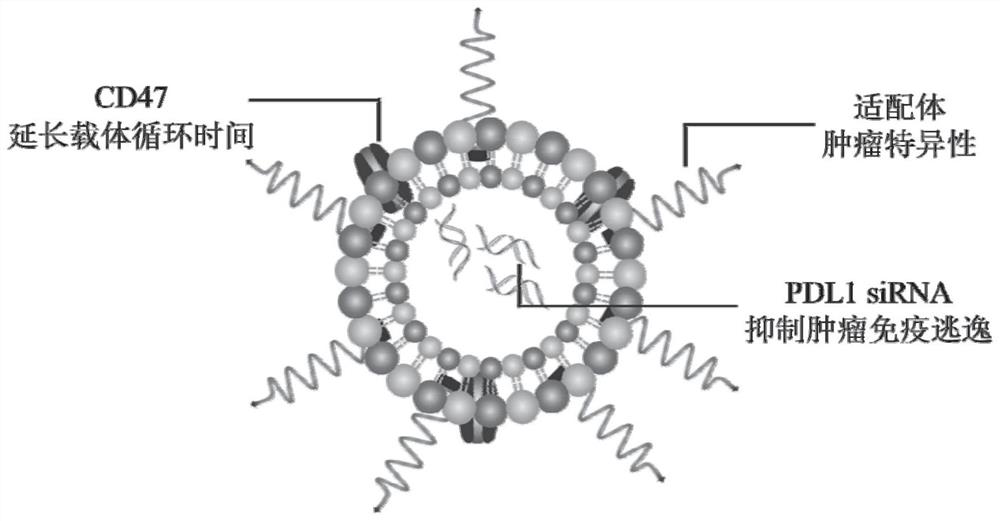
[0052]In some more specific embodiments, the mass ratio of the siRNA, cationic liposomes and nanovesicles is 1: (3-5): (30-160).
[0053] In some more s...
Embodiment 1
[0129] (1) Add erythrocytes to 1×PBS, centrifuge at 800g at 4°C for 5 minutes, collect erythrocytes, and repeat twice. Then red blood cells were added to 0.25×PBS, incubated on ice for 1 hour with shaking, 14000 rpm, and centrifuged at 4°C for 10 minutes to collect red blood cell membranes. Then add 0.25×PBS, shake and incubate on ice for 1h, centrifuge at 14000rpm, 4°C for 10min, and collect the red blood cell membrane. Then the erythrocyte membrane aqueous solution, absolute ethanol and chloroform volume ratio are 1: 5: 10, stir, filter, rotary steam, dry, add water and ultrasonically disperse, prepare the erythrocyte membrane with better water dispersibility; Wherein, erythrocyte and 0.25× The volume ratio of PBS is 1:9.
[0130] (2) Put the collected erythrocyte membrane into an ultrasonic breaker for ultrasonic crushing, the conditions are 60W power, 25KHz frequency, horn model Φ2, ultrasonication for 1min. Then, through an Avantiminiextruder liposome extruder equipped ...
Embodiment 2
[0133] (1) Add erythrocytes to 1×PBS, centrifuge at 800g at 4°C for 5 minutes, collect erythrocytes, and repeat twice. Then red blood cells were added to 0.25×PBS, incubated overnight with shaking on ice, centrifuged at 14000 rpm, 4°C for 10 min, and the red blood cell membrane was collected. Then add 0.25×PBS, shake and incubate on ice for 1h, centrifuge at 12000rpm, 4°C for 20min, and collect the red blood cell membrane. Then, the volume ratio of erythrocyte membrane aqueous solution, absolute ethanol and chloroform is 1:5:10, stirred, filtered, rotary evaporated, dried, added with water and ultrasonically dispersed to prepare erythrocyte membrane with better water dispersibility. Wherein, the volume ratio of red blood cells and 0.25×PBS is 1:9.
[0134] (2) Place the collected erythrocyte membrane in an ultrasonic breaker for sonication, the conditions are 20W power, 20KHz frequency, horn model Φ2, ultrasonic 5min, and then use Avantiminiextruder liposome extruder, equippe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com