Kit and method for synchronously detecting multiple genital tract pathogens
A synchronous detection and kit technology, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as low sensitivity, achieve strong specificity, improve detection efficiency, and good detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Kit
[0049] The kit includes two nucleic acid reaction solutions, namely nucleic acid reaction solution A and nucleic acid reaction solution B.
[0050] Nucleic acid reaction solution A is a quadruple nucleic acid reaction solution of Neisseria gonorrhoeae / Ureaplasma urealyticum / Chlamydia trachomatis / human internal reference, specifically including the following components:
[0051] (1) It consists of a pair of primers for detecting Neisseria gonorrhoeae and a probe for detecting Neisseria gonorrhoeae, the 5' end of the probe is marked with a CY5 fluorescent reporter group, and the 3' end is marked with a BHQ-2 fluorescence quencher group;
[0052] (2) It consists of a pair of primers for detecting Ureaplasma urealyticum and a probe for detecting Ureaplasma urealyticum. The 5' end of the probe is labeled with a FAM fluorescent reporter group, and the 3' end is labeled with a BHQ-1 fluorescence quencher killing group;
[0053] (3) It consists of a pair o...
Embodiment 2
[0065] Embodiment 2: the detection of pathogen
[0066] (1) Extract the nucleic acid of the sample collected
[0067] The samples collected clinically (such as vaginal swab samples) are extracted with the nucleic acid extraction kit purchased by Beijing Tiangen Biotechnology Co., Ltd. to extract the genomic DNA of the samples.
[0068] (2) Using the kit of Example 1, the extracted nucleic acid and the nucleic acid reaction solution A in the kit and the rapid fluorescent quantitative PCR premixed reagent are composed of reaction system A according to Table 2, and the extracted nucleic acid and the nucleic acid in the kit Reaction solution B and rapid fluorescent quantitative PCR premixed reagents were used to form reaction system B according to Table 2.
[0069] Table 2
[0070] Reaction System A Reaction System B The final concentration of the substance in the system 2×FastFire qPCR PreMix 2×FastFire qPCR PreMix 1× Neisseria gonorrhoeae probe Myc...
Embodiment 3
[0085] Embodiment 3: Sensitivity experiment
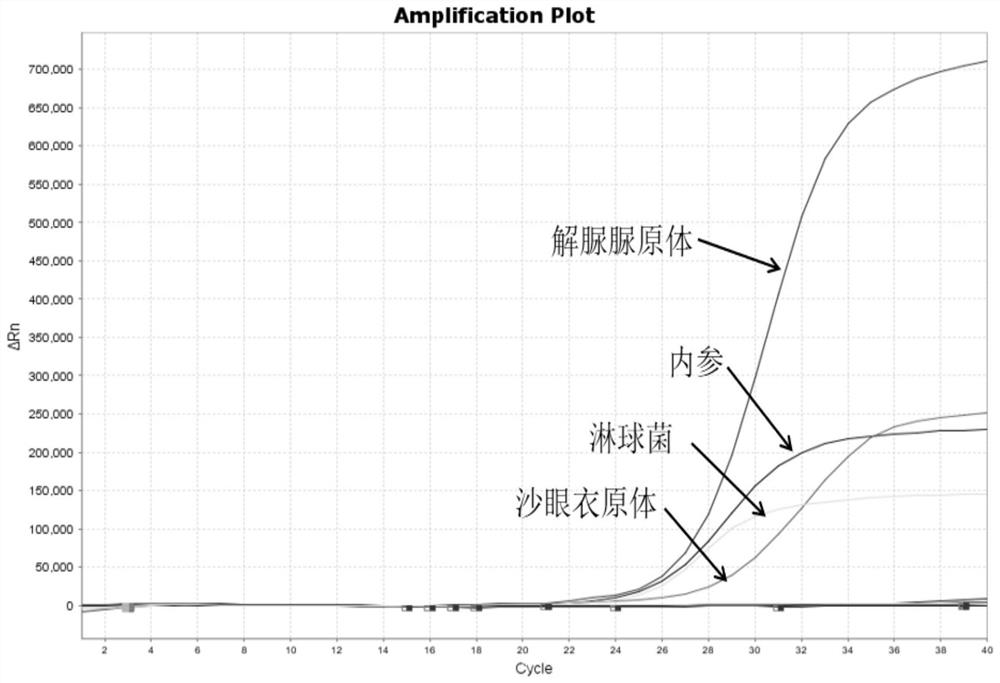
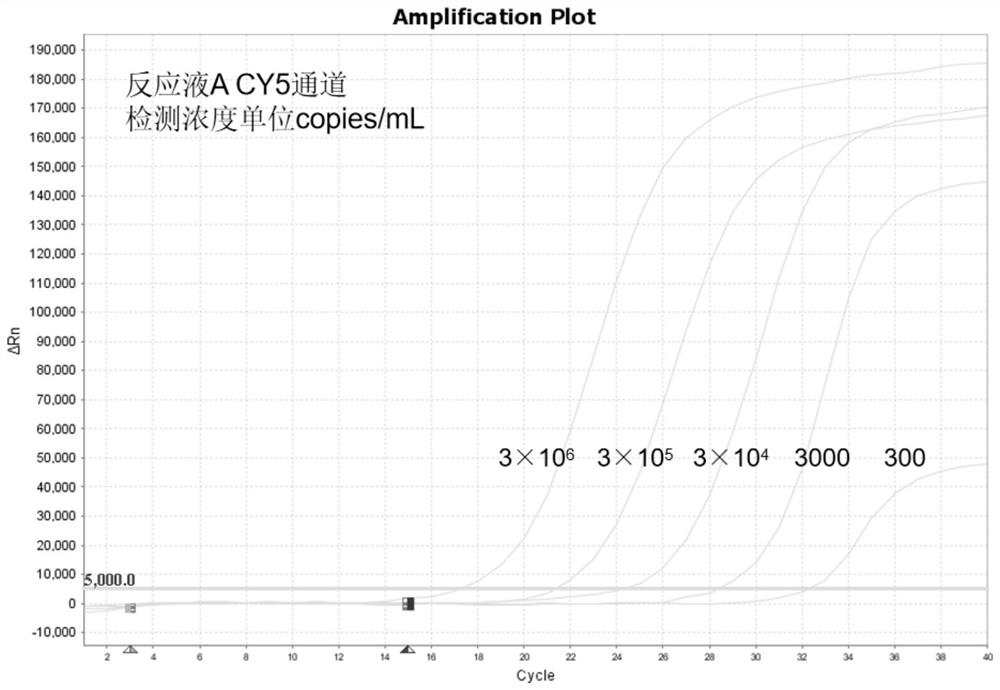
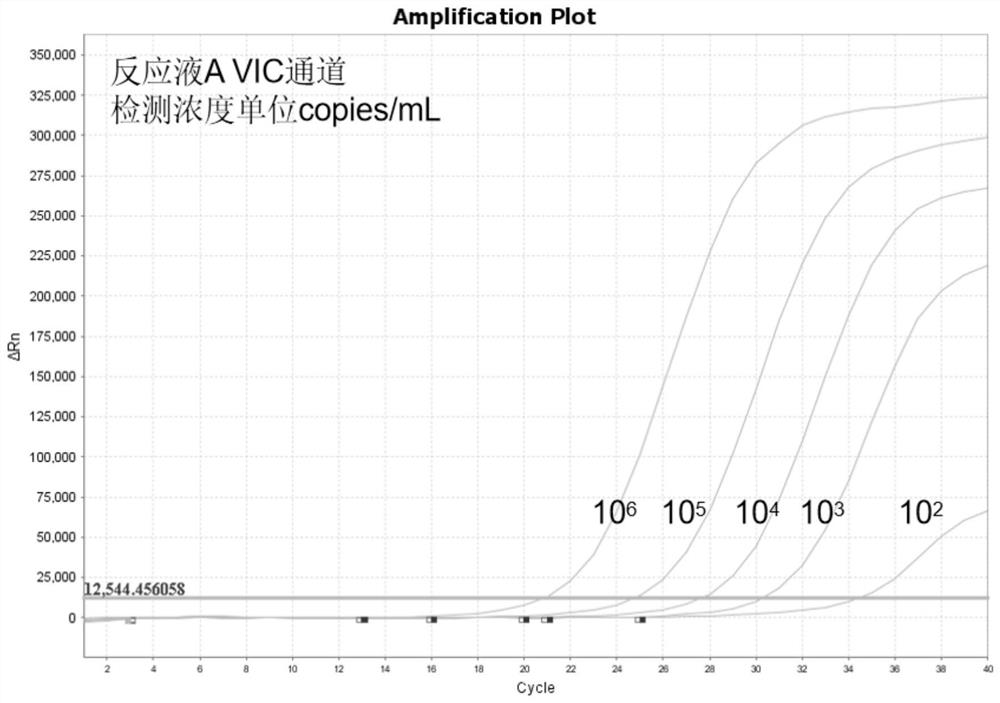
[0086] The positive reference products of nucleic acid reaction solution A are the DNA of Neisseria gonorrhoeae (NG), Ureaplasma urealyticum (UU), Chlamydia trachomatis (CT) and the DNA of purchased cell lines. The positive reference substances of nucleic acid reaction solution B are Mycoplasma hominis (MH), Mycoplasma genitalium (MG), NDA of herpes simplex virus type 2 (HSV2) and DNA of purchased cell lines. Using the kit of Example 1, the sensitivity of the kit was tested according to the detection method of Example 2.
[0087] For test results, see Figure 2 to Figure 4 and Figure 6 to Figure 8 , the results show that: this kit has good sensitivity, in nucleic acid reaction solution A, Neisseria gonorrhoeae (NG) can realize the reference concentration detection of 300copies / mL, Ureaplasma urealyticum (UU) and Chlamydia trachomatis (CT) can realize 100copies / mL reference substance concentration detection. Mycoplasma hominis ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com