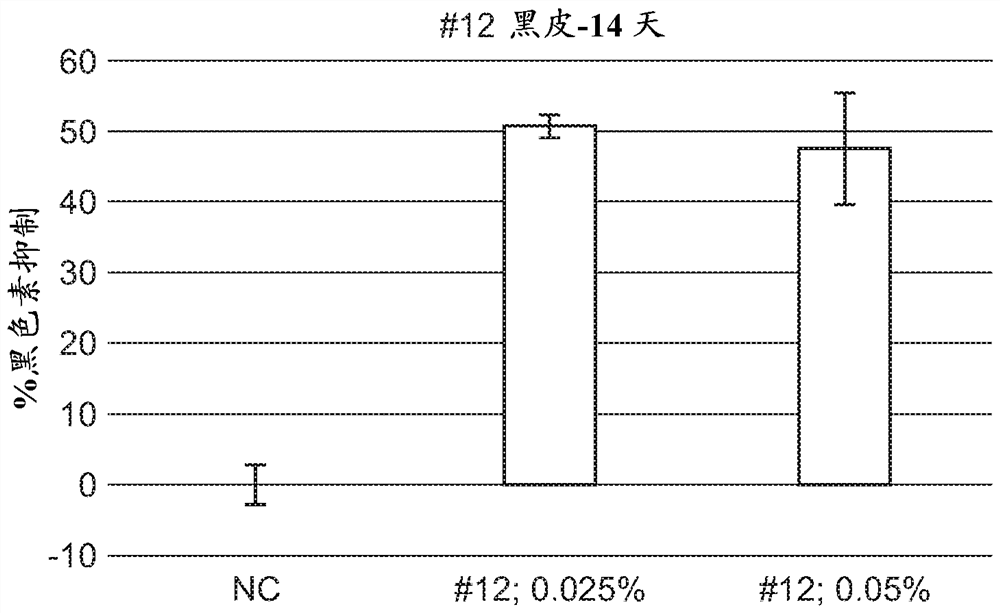
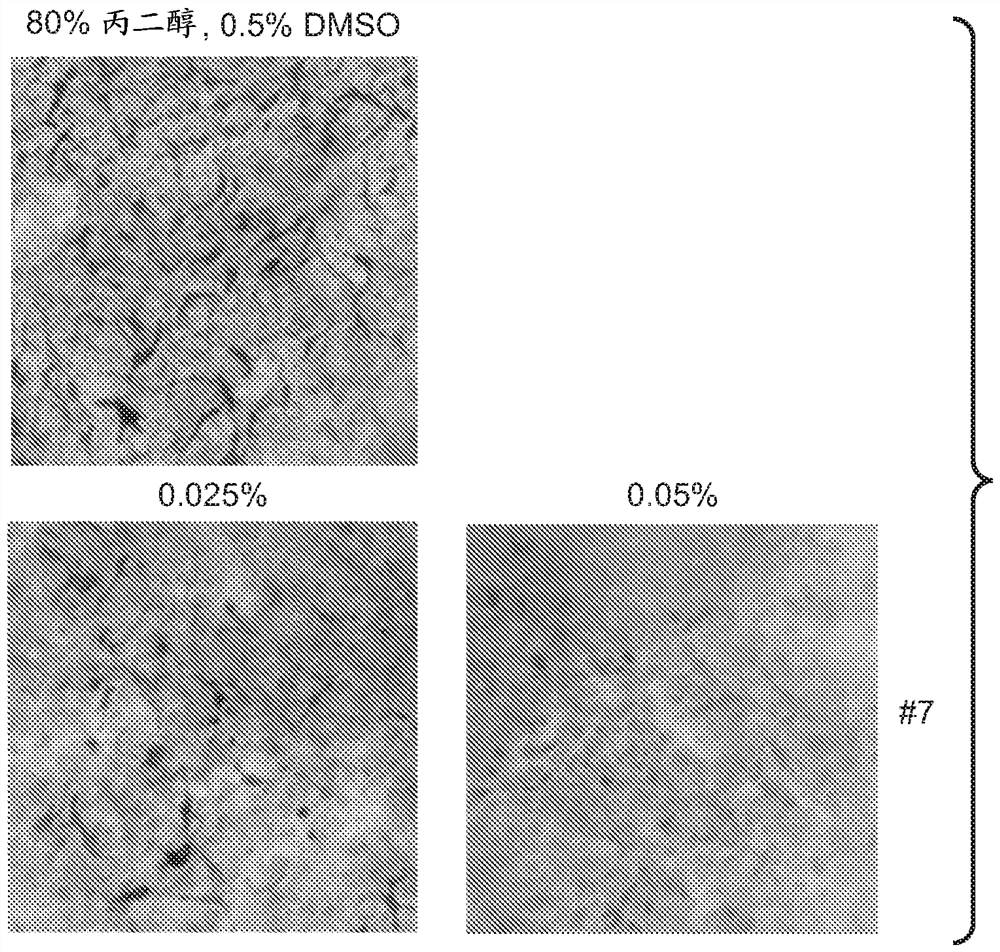
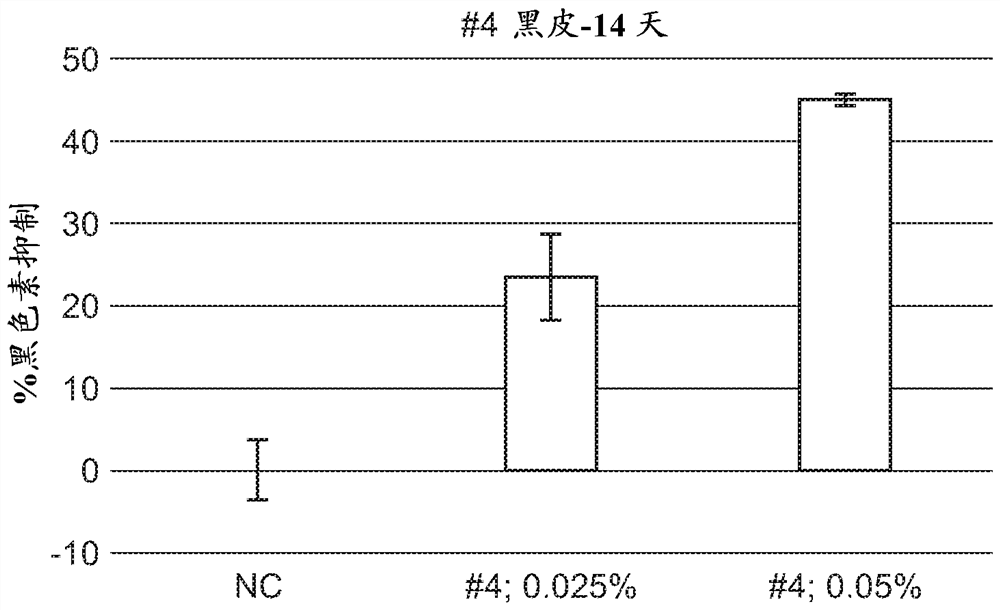
A series of compounds for treatment of skin diseases and other conditions
A compound, pyridyl technology, applied in skin diseases, effective ingredients of hydroxyl compounds, drug combinations, etc., can solve problems such as adverse side effects, less skin whitening agents, and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1: General and Specific Methods for the Synthesis of Compounds of Formula I
[0147] Compounds of formula I were prepared generally as described in Scheme 1, using a substituted benzyl phosphonate as R for purposes of illustration. Phosphonate reagents were prepared following the Arbuzov reaction with the corresponding benzyl bromide treated with triethyl phosphite. Briefly, 1,5-diene compounds of formula I are prepared from the corresponding phosphonates with the appropriate ketones by the Wittig reaction. The coupled diene product is then treated with methylmagnesium iodide for demethylation to yield the desired phenolic diene.
[0148]
[0149] Representative procedure for Scenario 1:
[0150] synthesis( E / Z )-4-(2,6-dimethylhept-1,5-dienyl)phenol (1):
[0151] General / typical procedure for the synthesis of phosphonates via the Albuzov reaction: 4-Methoxybenzyl bromide (20 g, 100 mmol) in P(OEt) 3 (23 mL, 140 mmol) was refluxed for 5 hours, then b...
Embodiment 2
[0159] Example 2 : General and specific methods of synthetic formula II compounds
[0160] Compounds of formula II are prepared generally as described in Scheme 2, using substituted bromobenzenes as R for illustrative purposes. Briefly, 1,6-diene compounds of formula II are prepared from the corresponding Grignard reagents with appropriate aldehydes by Grignard addition reactions. Grignard reagents were prepared from the corresponding aryl bromides using activated Mg chips. Then use POCl 3 Treatment of the coupled product for dehydration followed by demethylation with MeMgI affords the desired phenolic diene.
[0161] Scenario 2
[0162]
[0163] Representative procedure for Scheme 2: Synthesis ( E )-4-(3,7-dimethyloct-1,6-dienyl)phenol (5):
[0164] General / Typical Procedure for Grignard Addition Reaction: 4-Bromoanisole (11.2 g, 60 mmol) in THF (60 mL) was added dropwise to THF ( 10 mL) of magnesium turnings (1.9 g, 80 mmol) and iodine (a little). After the addit...
Embodiment 3
[0195] Example 3 : General and specific methods of synthetic formula III compounds
[0196] Compounds of formula III are prepared generally as described in Scheme 4, using a substituted benzyl phosphonate as R for illustrative purposes. Phosphonate reagents were prepared following the Albuzov reaction with the corresponding benzyl bromide treated with triethyl phosphite. Briefly, 1,7-diene compounds of formula III are prepared from the corresponding phosphonates with appropriate ketones by Wittig reactions. The coupled olefinic product is then treated with methylmagnesium iodide for demethylation to yield the desired phenolic diene.
[0197] Option 4
[0198]
[0199] General / typical procedure for the synthesis of phosphonates via the Albuzov reaction: p-methoxybenzyl bromide (10.5 g, 52.2 mmol) in P(OEt) 3 (25 mL) was refluxed for 5 hours, then bromoethane and excess triethyl phosphite were removed by distillation under reduced pressure. The residue afforded 12.8 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com