Abiraterone acetate solid self-microemulsion and preparation method thereof
A technology of abiraterone acetate and self-microemulsion, which is applied in the directions of pharmaceutical formulations, emulsion delivery, medical preparations with inactive ingredients, etc., can solve the problems of inconvenient transportation, preservation and taking of liquid preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The selection of each composition of embodiment 1 abiraterone acetate solid self-microemulsion
[0041] 1.1. Solubility test
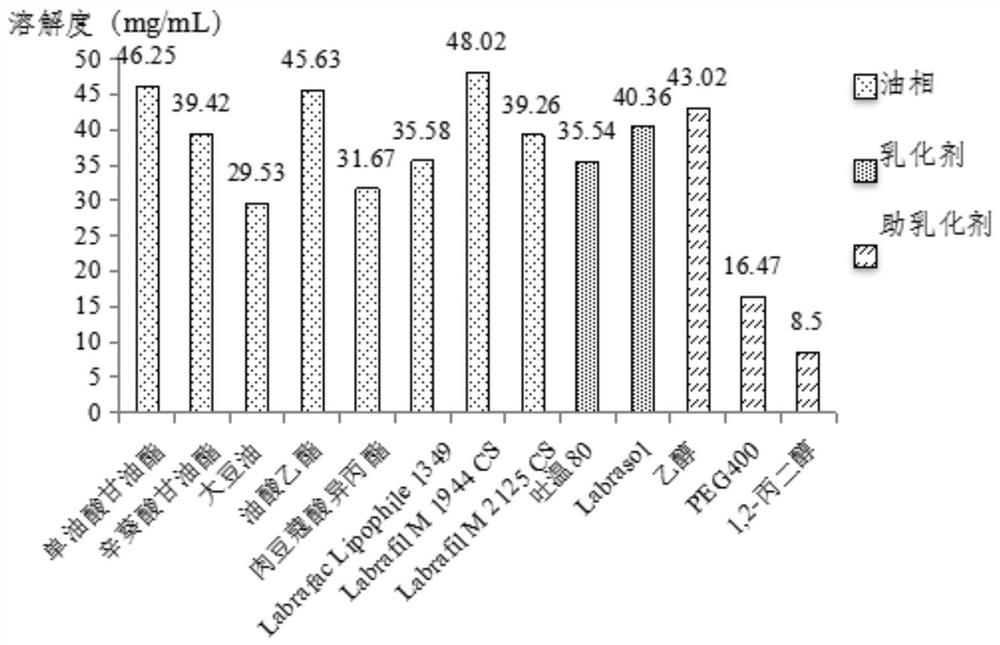
[0042] Take multiple 10mL EP tubes, add 1g of abiraterone acetate standard to each EP tube, add 5mL of different oils, emulsifiers and co-emulsifiers to different EP tubes and seal them. Fix the EP tube in a constant temperature water bath shaker, shake it for 48 hours at a water temperature of 37°C and 60 rpm, and then place it in a centrifuge for 10 minutes at a speed of 5000 rpm. Take out the centrifugate and filter it with a 0.45 μm microporous membrane, then dilute its volume 5 times with acetonitrile, and enter the liquid phase to detect the solubility of abiraterone acetate in different oils and surfactants (see figure 1 ).
[0043] Among them, the chromatographic conditions are:
[0044] Chromatographic column: Promosil C18 (4.6mm×250mm, 5μm)
[0045] Detector: UV 254nm
[0046] Flow rate: 1.2mL / min
[0047] Mobile phase: phosphate...
Embodiment 2
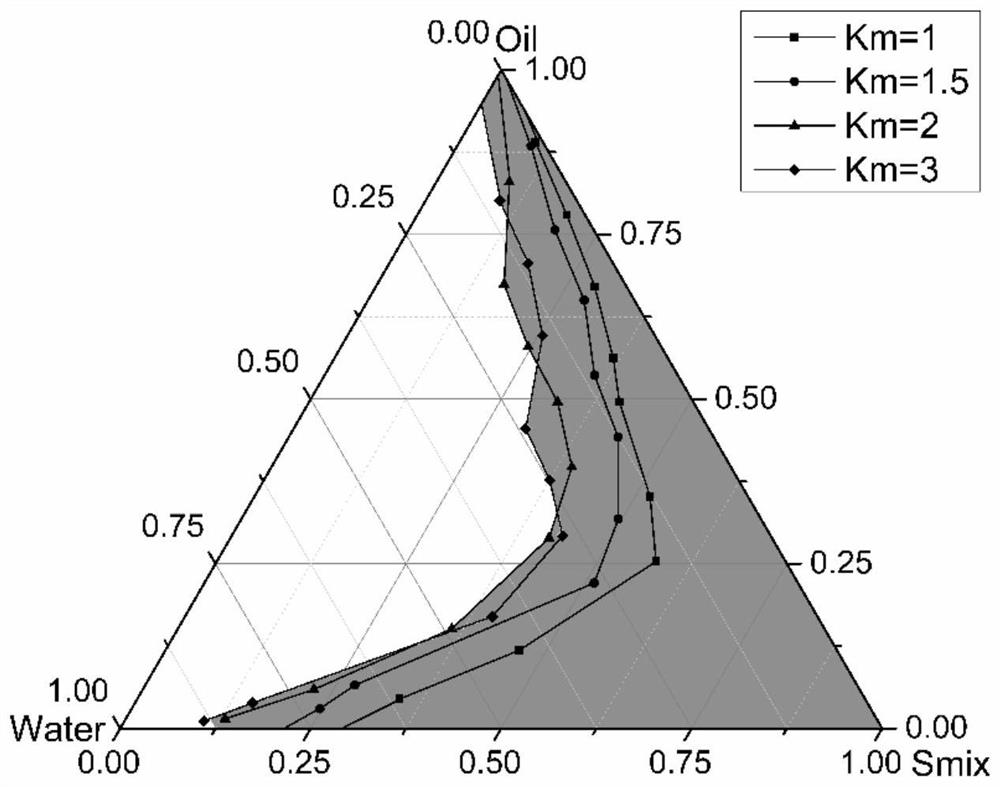
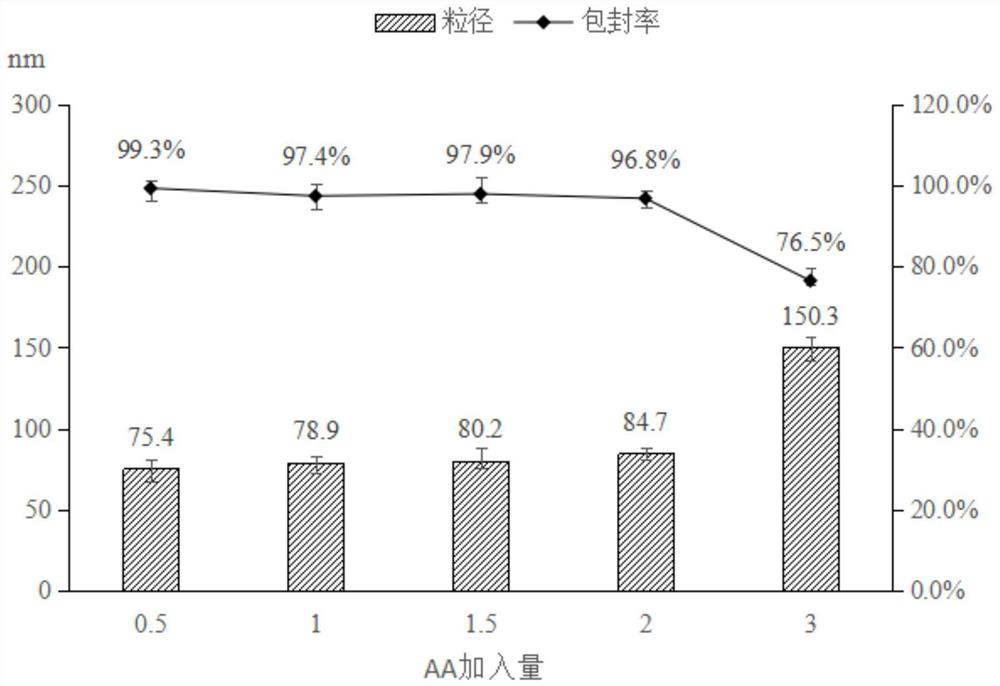
[0068] The ratio optimization of each component in the Abiraterone acetate solid self-microemulsion system of embodiment 2
[0069] In this experiment, the amount of oil phase, the ratio of surfactant to co-surfactant / Km, and the amount of drug added were screened, and the optimal system was obtained based on particle size, appearance and stability.
[0070] 2.1 Amount of oil phase
[0071] The ratio of fixed surfactant and co-surfactant is 2: 1 (v / v), adding blank liquid self-microemulsion preparation (oil phase, emulsifier and co-emulsifier) total mass 10%, 15%, 20%, 25%, 30% oil phase, the three are mixed evenly to make a blank self-microemulsion preparation. Take 1 g of the blank preparation and add 10 mL of deionized water to dilute, observe the stability of the diluent within 48 hours, and take a small amount of the diluent to measure the particle size and its distribution (see Table 3).
[0072] The influence of table 3 oil phase dosage on preparation properties
...
Embodiment 3
[0091] At room temperature, weigh 0.263 g of ethanol and 0.534 g of caprylic capric acid polyethylene glycol glyceride (Labrasol) and mix them in a small beaker to obtain a surfactant mixture.
[0092] Add 10.2 mg of abiraterone acetate to the surfactant mixture obtained above and mix evenly, and ultrasonically assists in accelerating the dissolution of the drug. After the drug is completely dissolved, add 0.207 g of glyceryl monooleate and shake well to obtain acetic acid Abiraterone Liquid Self Microemulsion.
[0093] Take 1.5g of abiraterone acetate liquid self-microemulsion and 1.5g of cross-linked PVP to mix and absorb, and obtain 3g of abiraterone acetate solid self-microemulsion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com