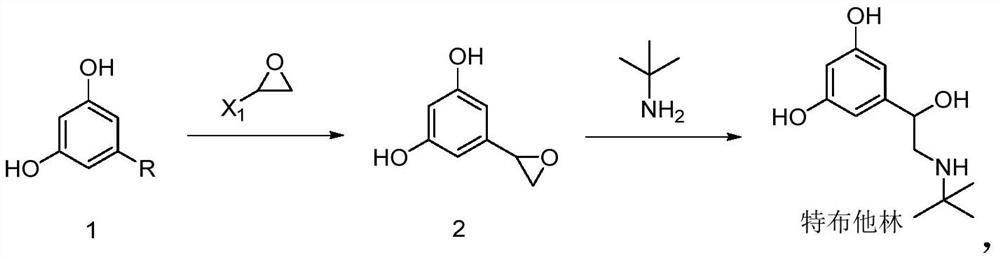
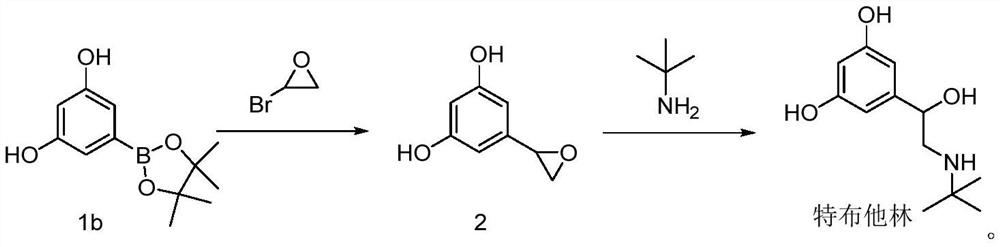
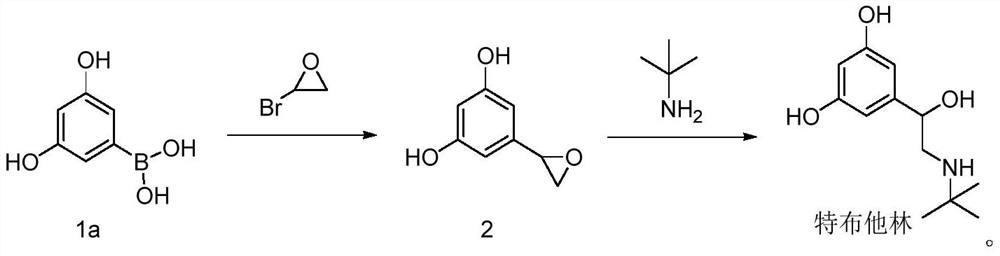
Preparation method of terbutaline
A technology for terbutaline and a compound, which is applied in the field of preparation of terbutaline, can solve the problems of expensive and unobtainable raw materials, lack of economy, long synthesis route and the like, and achieves easy control of the reaction process, shortened reaction steps and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Preparation of compound 1b (3,5-dihydroxybenzeneboronic acid pinacol ester)
Embodiment 1-1
[0065]
[0066] Under nitrogen protection, 19.0g 5-bromoresorcinol, 28.0g biboronic acid pinacol ester, 30.0g potassium acetate and 2.2g Pd(dppf)Cl were mixed 2 Dissolve in 150 mL of 1,4-dioxane, heat to 100 °C, and monitor by TLC to detect no 5-bromoresorcinol. The mixture was cooled to room temperature, diluted with toluene, filtered to remove insolubles, the filtrate was washed with an equal volume of water, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to obtain 21.1 g of compound 1b with a yield of 89.0%, HPLC purity 95.8%.
Embodiment 1-2
[0068]
[0069] Under nitrogen protection, 14.0g 5-chlororesorcinol, 28.0g biboronic acid pinacol ester, 25.0g sodium acetate and 2.2g Pd(dppf)Cl were mixed 2 Dissolved in 150 mL DMSO, heated to 120°C, and monitored by TLC for the absence of 5-chlororesorcinol. It was cooled to room temperature, diluted with toluene, filtered to remove insolubles, the filtrate was washed with an equal volume of water, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to obtain 19.5 g of compound 1b with a yield of 82.3%. HPLC purity 95.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com