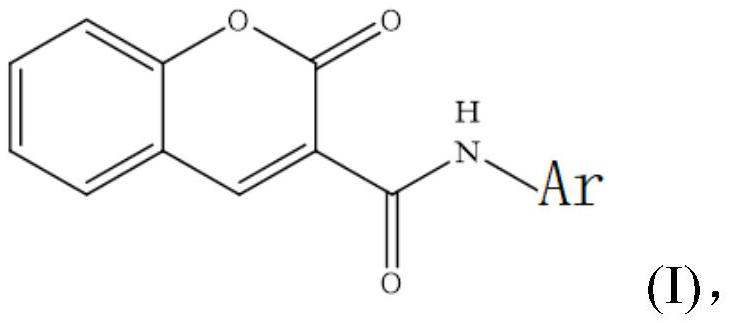
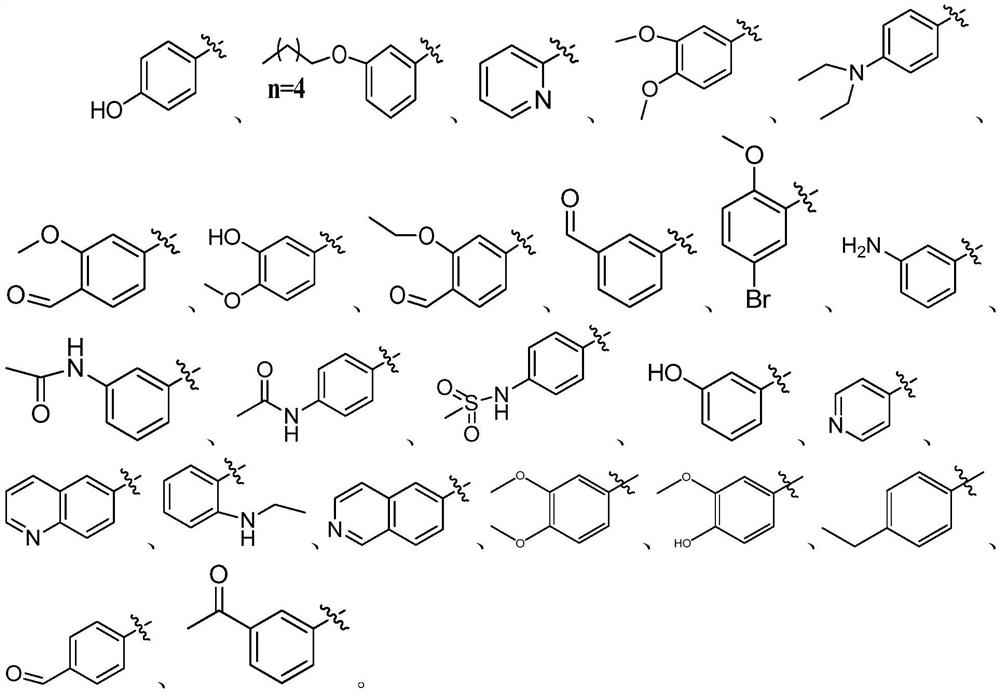
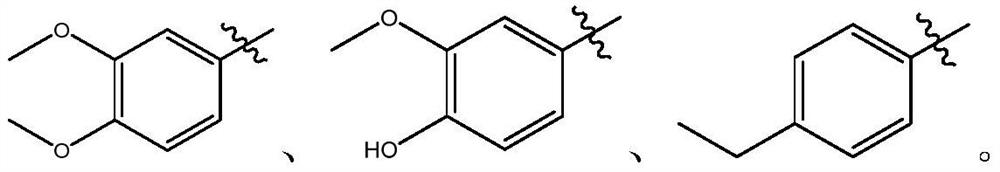
Coumarin 3-formamide neuraminidase inhibitor as well as preparation method and application thereof
A neuraminidase and formamide technology, applied in the field of biomedicine, can solve the problems of expensive raw materials for production of Tamiflu, complex synthesis process, etc., and achieves good neuraminidase inhibitory activity, simple synthesis method, and excellent neuraminic acid. Effect of enzyme inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] Described preparation method specifically comprises the following steps:
[0039] (1) forming a reaction system with salicylaldehyde and diethyl malonate, and obtaining the intermediate of formula (II) through aftertreatment after the reaction;
[0040] (2) The formula (II) intermediate obtained in step (1) and the substituted aniline are dissolved in an organic solvent to form a reaction system, and after the reaction, the coumarin 3-carboxamides shown in the formula (I) are obtained through post-treatment. agent;
[0041] In step (1), piperidine and acetic acid are used as catalysts, and absolute ethanol is used as the organic solvent.
[0042] In step (1), the temperature of the reaction system is 77-79°C, preferably 78°C, and the reaction time is 8-10h, preferably 8h.
[0043] In step (1), the post-treatment process specifically includes: taking out the reaction system for cooling, adding 50 ml of ice water to the reaction system in an ice-water bath for dilution....
Embodiment 1
[0052] A preparation method containing coumarin 3-carboxamide neuraminidase inhibitor, its structural formula is as follows:
[0053]
[0054] Concrete synthetic steps are as follows:
[0055](1) Accurately measure 1.83g (15mmoL) of salicylaldehyde and 2.88g (18mmoL) of diethyl malonate with a graduated cylinder, pour them into a 100ml round bottom flask, pour 30mL of absolute ethanol, and stir. 0.15 mL of piperidine was added, and 2 drops of acetic acid were added dropwise. Place in an oil bath and heat and stir at 78° C. for 8 hours. After the reaction is over, dilute the reaction system with 50 ml of ice water in an ice-water bath, and a large amount of solids are produced in the system solution. Filter the solid to obtain a filter cake, wash the filter cake with ice water, and then dry the filter cake at room temperature to obtain the intermediate of formula (II).
[0056] (2) Take 0.64g (3mmoL) of the intermediate of formula (II) and 0.55g (3.6mmoL) of 3,4-dimethoxya...
Embodiment 2
[0086] A preparation method of coumarin 3-carboxamide neuraminidase inhibitor, its structural formula is as follows, and it is prepared by a method similar to Example 1.
[0087] N-(3-methoxy-4-hydroxyphenyl)-2-oxo-2H-chromene-3-carboxamide
[0088]
[0089] Yellow solid, 5% yield, IC 50 The value is 35.56 μM.
[0090] 1 H NMR (500MHz, DMSO-d 6 )δ13.37(s,1H),9.25(s,1H),8.88(s,1H),7.62(d,J=5.9Hz,1H),7.38(t,J=7.0Hz,1H),6.93( m,5H), 3.81(s,3H). 13 C NMR (125MHz, DMSO-d 6 )δ161.43, 160.64, 147.74, 147.62, 141.42, 133.15, 132.78, 119.83, 119.45, 116.92, 114.03, 113.11, 112.94, 108.74, 40.49.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com