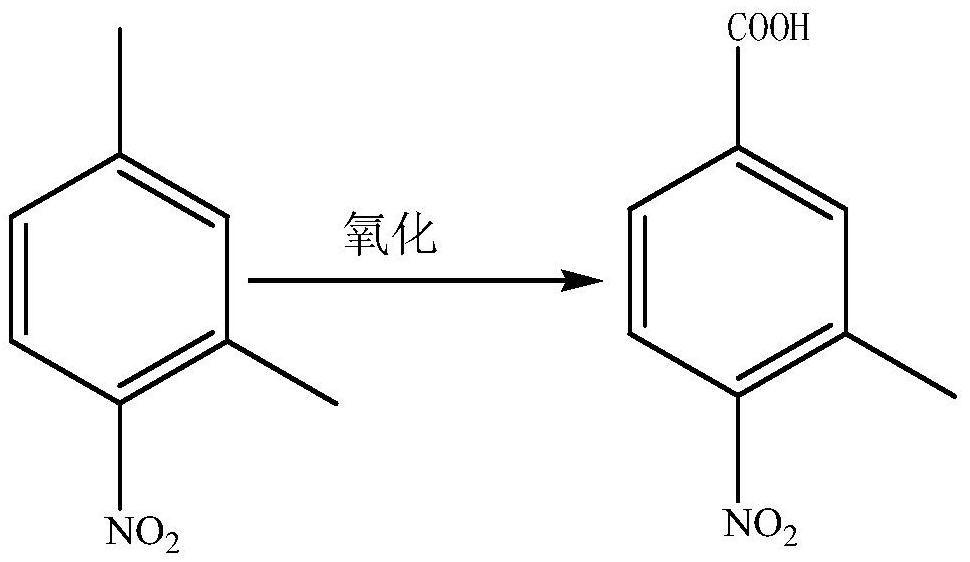
Preparation method of 3-methyl-4-nitrobenzoic acid
A technology of nitrobenzoic acid and methyl, which is applied in the field of preparation of antihypertensive drug telmisartan intermediates, can solve the problems of low technical selectivity and low conversion rate, and achieve stable reaction, easy control, and conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] (1) Preparation of 3-methyl-4-nitrobenzoic acid
[0013] 1. Add 300mL of acetonitrile into the photoreactor (with a filter tube with a filter wavelength less than 380nm), blow in oxygen from below, then add 5g of 2,4-dimethylnitrobenzene, 380mg of catalyst hematoporphyrin, and 2g of NiO , Turn on the high-pressure mercury lamp, react at 30°C for 5 hours, filter, spin the filtrate to recover the solvent acetonitrile, add water, stir to dissolve, acidify to pH=3.8, filter to obtain 3.95g of 3-methyl-4-nitrobenzoic acid, purity 96.7 %.
[0014] 2. Add 300mL of acetonitrile into the photoreactor (with a filter tube with a filter wavelength less than 380nm), blow in oxygen from below, then add 5g of 2,4-dimethylnitrobenzene, and 395mg of catalyst hematoporphyrin monomethyl ether , NiO 2g, turn on the high-pressure mercury lamp, react at 30°C for 5h, filter, spin the filtrate to recover the solvent acetonitrile, pour the residue into water, acidify to pH=3.8, and filter to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com