A preparation method and application of fully human CAR-T cells targeting CD276
A CD276, VH-CDR1 technology, applied in the field of CAR-T cell preparation, can solve problems such as short survival, and achieve the effect of high killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
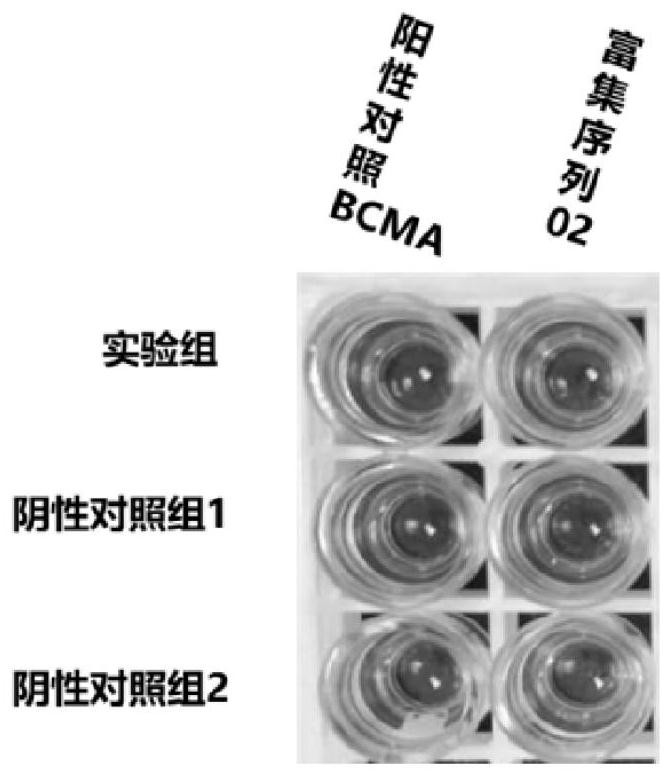
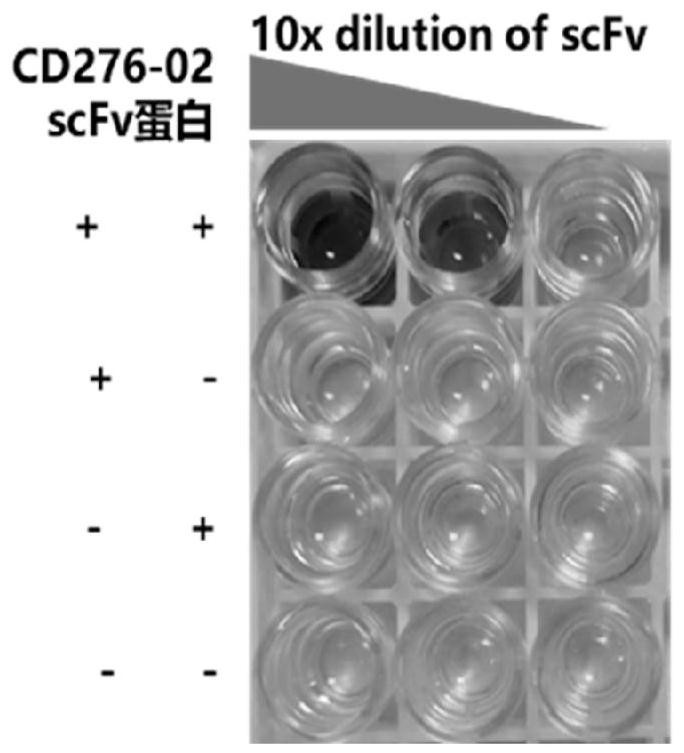
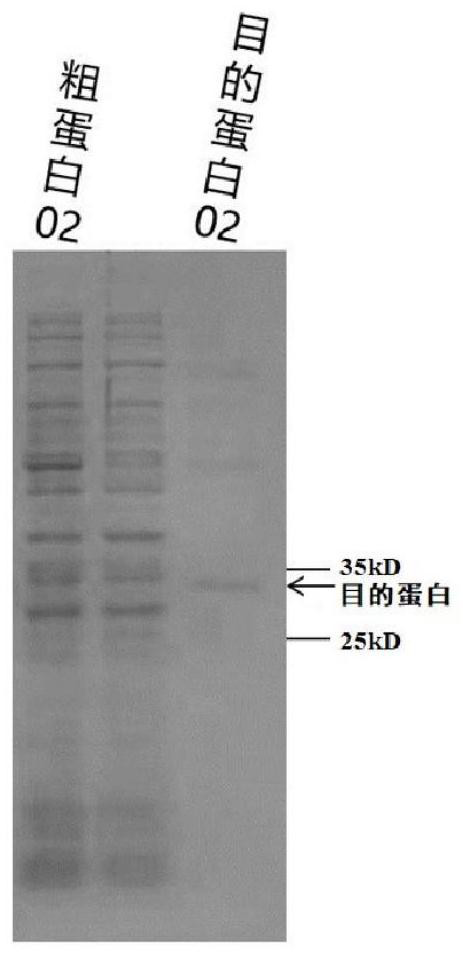
[0116] Example 1 Screening of scFv targeting CD276
[0117] 1. Experimental condition setting
[0118] Experimental group: CD276 antigen + CD276-Phage
[0119] Control group 1: other non-biotin antigen (PRPS1)+CD276-Phage
[0120] Experimental group 2: no antigen + CD276-Phage
[0121] 2. Experimental method
[0122] After four rounds of screening to enrich specific binding antibody sequences, the total amount of phage input, the amount of added antigen, and reaction time were changed in each round.
[0123] The final result is to judge the enrichment by calculating the number of phages with infectious ability contained in every 100 μl of 0.1M HCl (PH=2.0) eluate in the experimental group and the control group.
[0124] 3. Screening result analysis
[0125] Enrichment occurred in the third round of screening, and the ratio of the number of phage eluted in the experimental group (antigen-antibody specific binding) to the control group (antigen-antibody non-specific binding...
Embodiment 3
[0166] Example 3 Preparation of fully human CD276 CAR-T cells
[0167] 1. Steps
[0168] (1) Prepare PBMC cells
[0169] Take peripheral blood from healthy people, centrifuge and save autologous plasma for later use, dilute remaining blood cells with an equal volume of normal saline, add to the upper layer of lymphocyte separation medium, centrifuge, absorb middle buffy coat cells, add normal saline to wash, centrifuge and discard supernatant, Instantly.
[0170] (2) Construction of the shuttle plasmid MSCV-M13B702 containing the CAR structure
[0171] a. Synthesize the CAR encoding nucleotide sequence targeting human CD276, the CAR encoding nucleotide sequence is shown in SEQ ID NO.17, wherein the nucleotide sequence of the heavy chain VH of the scFv targeting human CD276 is shown in SEQ ID NO .9, the nucleotide sequence of the light chain VL is shown in SEQ ID NO.10, the nucleotide sequence of the G4S short peptide is shown in SEQ ID NO.12, and the nucleotide sequence of ...
Embodiment 4
[0195] Example 4 In vivo functional verification of fully human CD276 CAR-T cells
[0196] 1. Steps
[0197] 5*10 of the fluorescent signal will be carried 5 SKOV3-luc-GFP was injected intraperitoneally into NCG mice, and the tumor formation in the peritoneal cavity of the mice was monitored by taking pictures every week by using small animal in vivo imaging. After the formation of abdominal tumors, fully human fhCD276-02CAR-T cells were injected intraperitoneally, and the control group was used Humanized CD276 CAR-T cells. Thereafter, tumor regression in the peritoneal cavity of the mice was observed by intravital imaging every week.
[0198] 2. Results
[0199] The result is as Figure 10 As shown, the CAR-T cells of the present invention can clear the xenograft tumor of mouse peritoneal ovarian cancer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com