Novel cell lysis buffer solution for detecting cell ubiquitin modified proteins
A lysis buffer, ubiquitin-like technology, applied in the field of new cell lysis buffer, can solve the problem of inability to detect proteins, and achieve the effect of reducing the number of repetitions, saving consumables and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
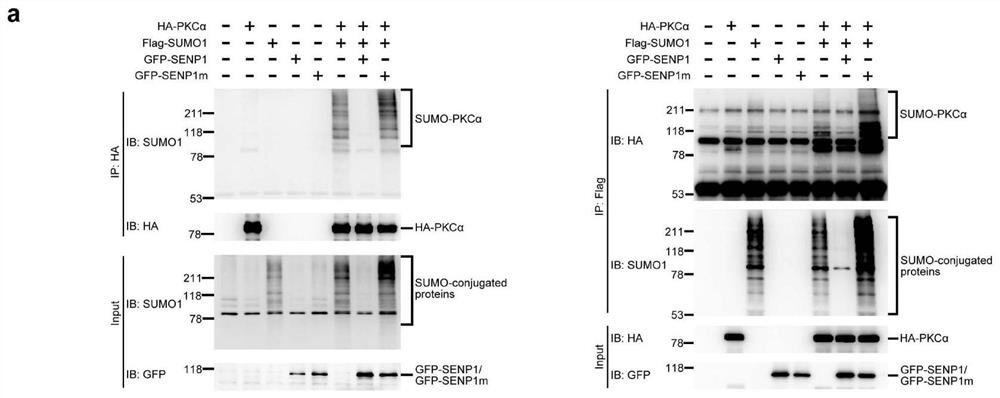
[0028] S1: 3x10 6 After the cultured CHO-K1 cells (hamster ovary cell substrain) were transfected with plasmids HA-PKC, Flag-SUMO1, GFP-SENP1 or GFP-SENP1m (SENP1 mutant inactivation) by conventional methods (using Lipofectamine 2000 transfection reagent) , continue to cultivate for 36-48 hours;
[0029] S2: Collect the transfected CHO-K1 cells into a 1.5ml centrifuge tube, centrifuge through a cell centrifuge (500g, 2 minutes), wash the cells once with PBS buffer (Phosphate Buffer Saline) after removing the supernatant, and centrifuge again ( 500g, 2 minutes), after the supernatant was removed, the cell pellet was collected in a 1.5ml centrifuge tube;
[0030] S3: Add 100 ul of the buffer solution 1 of the present invention into the above-mentioned centrifuge tube, fully oscillate and break up, place in a 95°C water bath for 15 minutes, then immediately take it out and place it on ice for 15 minutes;
[0031] S4: Add 900ul of the buffer solution 2 of the present invention t...
Embodiment 2
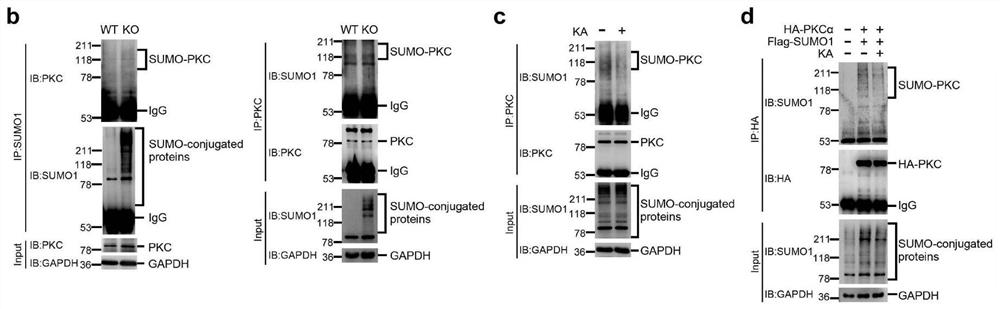
[0036] S1: Embryonic cells from wild-type mice (WT) or SENP1 (a deubiquitinase) knockout mouse (KO) ( figure 2 b), or rat spinal cord neuron cells stimulated with Kainate (KA, 200mM, 1min) (KA+)( figure 2 c) Centrifuge the cells with a cell centrifuge (500g, 2 minutes), remove the supernatant and wash the cells once with PBS buffer (Phosphate Buffer Saline), centrifuge again (500g, 2 minutes), remove the supernatant and then pellet the cells Collected in a 1.5ml centrifuge tube;
[0037] S2: Add 100 ul of the buffer solution 1 of the present invention into the above-mentioned centrifuge tube, oscillate fully, place in a water bath at 95°C for 15 minutes, then immediately take it out and place it on ice for 15 minutes;
[0038] S3: Add 900 ul of the buffer solution 2 of the present invention to the centrifuge tube, shake well and evenly, place on ice for use or store at -80°C;
[0039] S4: Add 1ug SUMO1 antibody or 1ug PKC antibody ( figure 2 b). Add 1ug of PKC antibody ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com