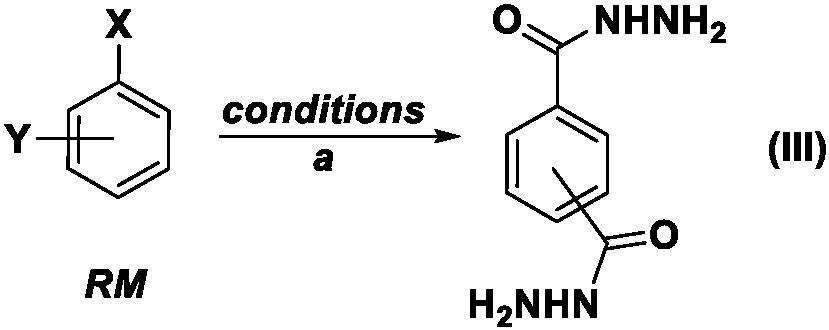
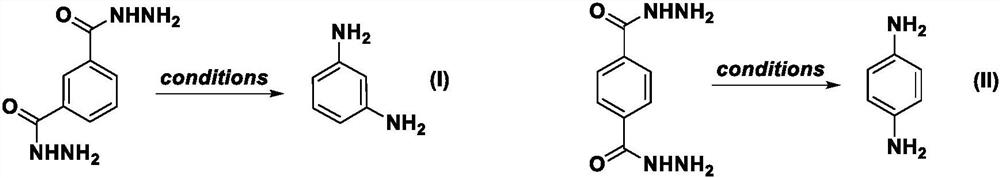Benzoyl hydrazine rearrangement method for preparing m-phenylenediamine and p-phenylenediamine
A technology of phthalohydrazide and phenylenediamine, applied in the field of organic functional new material chemicals, can solve the problems of process safety risk, environmental protection pressure, and no significant breakthrough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment one: the synthesis of m-phenylenediamine
[0063]
[0064] Under the protection of nitrogen, put 28 grams of isophthalic acid dihydrazide in 300 milliliters of 1N concentration hydrochloric acid solution, under high-speed shear stirring, the system is cooled to about 5 degrees Celsius, and 25 grams of sodium nitrite in 50 milliliters of aqueous solution is slowly join in. After half an hour, 200 ml of toluene was added, the system was shaken, the toluene organic phase was separated, and the process was repeated once. After the organic phases were combined, they were stirred and heated at 80°C, paying attention to the release of nitrogen from the reaction system, and an equal volume of 1N sodium hydroxide solution was added, and the mixed system was stirred overnight. The organic phase was separated, washed successively with a small amount of water and half-saturated sodium bicarbonate, dried over anhydrous sodium sulfate, filtered, all solvents were remov...
Embodiment 2
[0065] Embodiment two: the synthesis of m-phenylenediamine
[0066]
[0067] Under nitrogen protection, 19.4 grams of dimethyl isophthalate was placed in 150 milliliters of ethanol, 10 milliliters of hydrazine monohydrate was added, and the reaction solution was refluxed overnight and then concentrated to about a quarter of its volume. After the reaction solution was cooled to room temperature, gradually 10.3 grams of isophthalic acid bishydrazide solid were precipitated.
[0068] Referring to the operation of Example 1, 4.1 grams of m-phenylenediamine products were obtained from 10.3 grams of isophthalic acid bishydrazide for rearrangement reaction.
Embodiment 3
[0069] Embodiment three: the synthesis of m-phenylenediamine
[0070]
[0071] Under nitrogen protection, 16.7 grams of isophthalic acid was placed in 150 milliliters of ethanol, 10 milliliters of hydrazine monohydrate was added, and the reaction solution was refluxed for 8 hours to maintain azeotropic water removal. After the reaction solution was cooled to room temperature, isophthalic acid was gradually precipitated. Formic acid bishydrazide solid 10.1 g.
[0072] Referring to the operation of Example 1, 3.8 grams of m-phenylenediamine products were obtained from 10.1 grams of isophthalic acid bishydrazide for rearrangement reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com