Preparation method of dexmedetomidine hydrochloride degradation impurity
A technology of dexmedetomidine hydrochloride and dexmedetomidine is applied in the field of preparation of dexmedetomidine hydrochloride to degrade impurities, achieving the effects of reducing side reactions, low production cost and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment provides a preparation method for degrading impurities by dexmedetomidine hydrochloride, comprising the following steps:
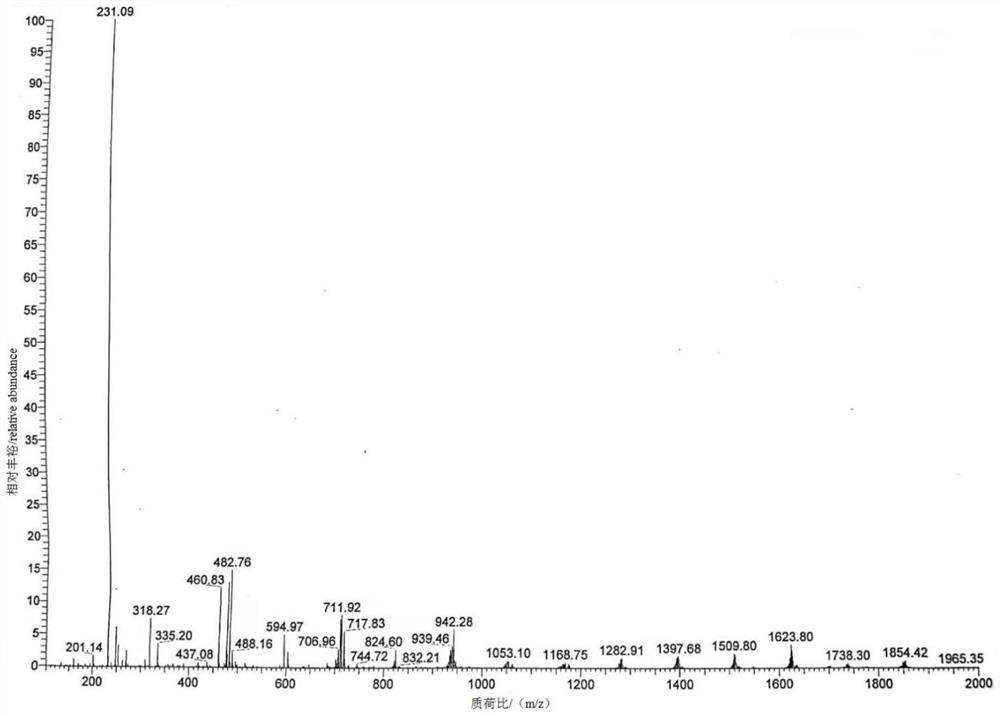
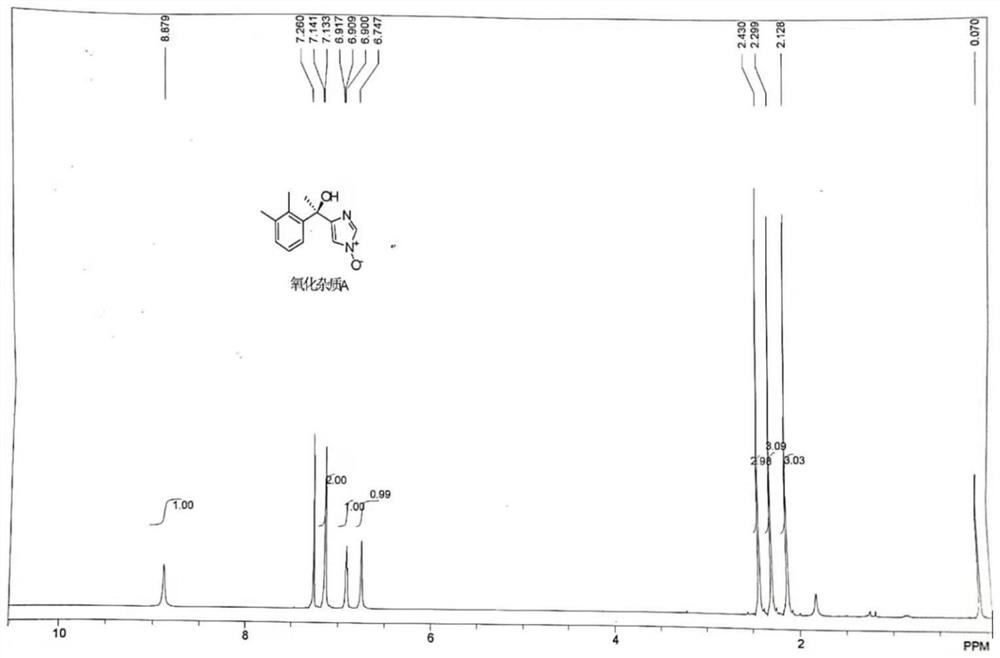
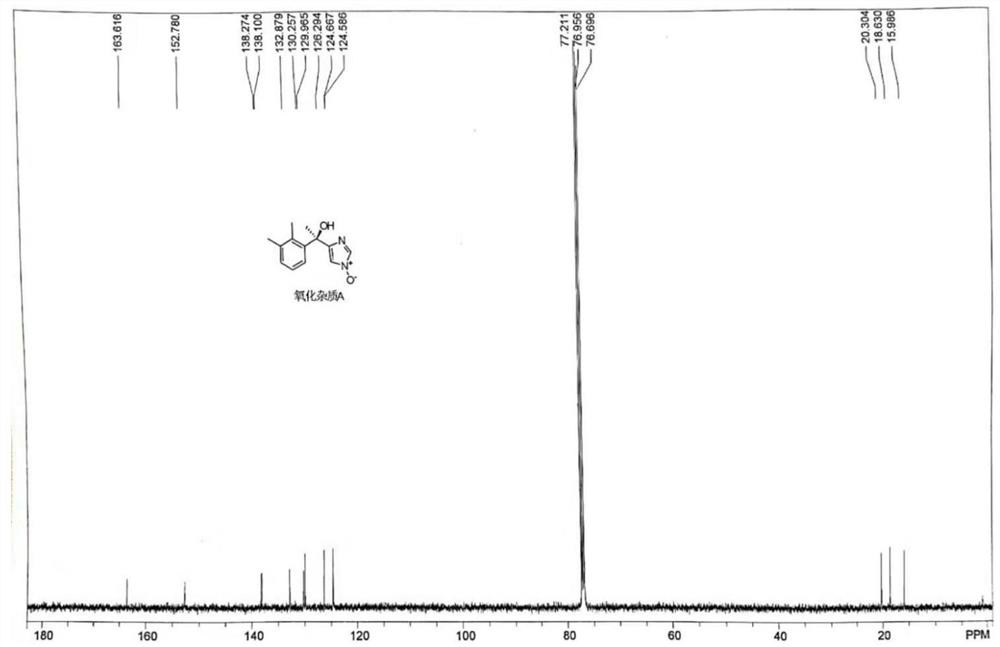
[0044] Step 1: Dissolve 20.1 g of dexmedetomidine in 140 mL of acetic acid, then add 46.2 g of NaBO 3 4H 2 O, temperature control and stirring reaction at 50° C. for 15 h to obtain a reaction solution containing the degraded impurities shown in formula (I);
[0045] Step 2: The temperature of the reaction solution was lowered to 20°C, 101mL of water and 140mL of ethyl acetate were added, stirred for 15min until uniform, the organic phase was collected after standing and liquid separation, and then the organic phase was dried over anhydrous sodium sulfate , and concentrated at a temperature of 40°C and a vacuum of 0.09MPa to obtain a crude product of degraded impurities. Finally, the crude product of degraded impurities was subjected to silica gel column chromatography (Jiangsu Lianhua Glass Instruments, a chromatography of 40mm×500m...
Embodiment 2
[0048] This embodiment provides a preparation method for degrading impurities by dexmedetomidine hydrochloride, comprising the following steps:
[0049] Step 1: Dissolve 20.1 g of dexmedetomidine in 101 mL of acetic acid, then add 92.4 g of NaBO 3 4H 2 O, temperature control and stirring reaction at 60° C. for 12 hours to obtain a reaction solution containing the degraded impurities shown in formula (I);
[0050] Step 2: The temperature of the reaction solution was lowered to 25°C, 161mL of water and 201mL of ethyl acetate were added, stirred for 20min until uniform, the organic phase was collected after standing and liquid separation, and then the organic phase was dried over anhydrous sodium sulfate , and at a temperature of 45° C. and a vacuum of 0.10 MPa, concentrate to obtain a crude product of degraded impurities. Finally, the crude product of degraded impurities is subjected to silica gel column chromatography (Jiangsu Lianhua Glass Instruments, with a specification of...
Embodiment 3
[0053] This embodiment provides a preparation method for degrading impurities by dexmedetomidine hydrochloride, comprising the following steps:
[0054] Step 1: Dissolve 20.1g of dexmedetomidine in 201mL of acetic acid, then add 61.5g of NaBO 3 4H 2 O, temperature control and stirring reaction at 55° C. for 15 h to obtain a reaction solution containing degraded impurities shown in formula (I);
[0055] Step 2: Cool the reaction solution to 30°C, add 201mL of water and 101mL of ethyl acetate, stir for 10min until uniform, let stand, and collect the organic phase after liquid separation, and then dry the organic phase over anhydrous sodium sulfate , and concentrated at a temperature of 42°C and a vacuum of 0.09MPa to obtain a crude product of degraded impurities. Finally, the crude product of degraded impurities was subjected to silica gel column chromatography (Jiangsu Lianhua Glass Instruments, a 40mm×500mm chromatography Column), carry out gradient elution with the volume r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com