SARS-CoV-2 Spike protein receptor binding domain dimer and application thereof
A sars-cov-2rbd, dimer technology, applied in the field of binding domain dimer and its application, can solve the problem of no clear evidence to determine the origin and intermediate host, achieve good neutralization activity and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
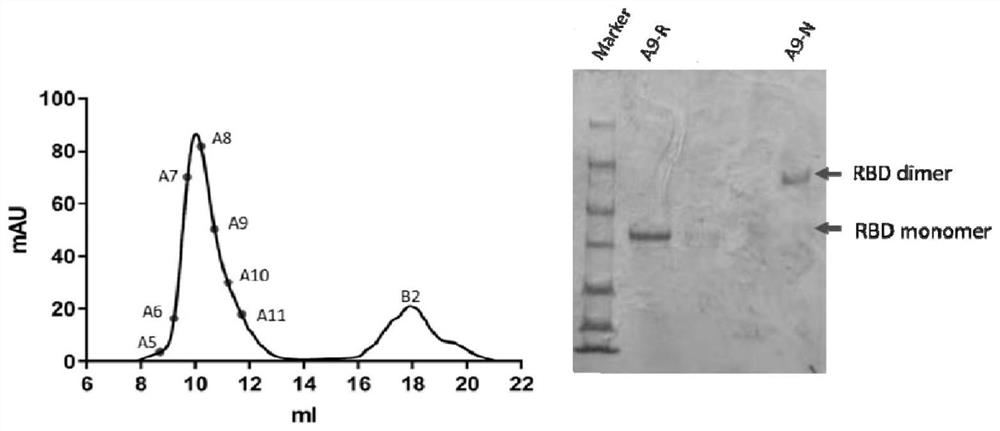
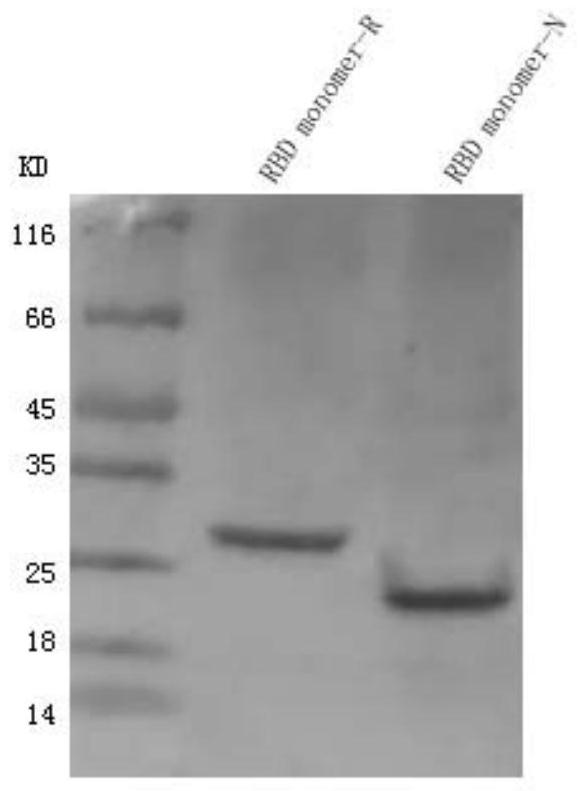
[0056] Embodiment 1, prepare SARS-CoV-2 RBD
[0057] 1. Preparation of dimers
[0058] 1. Use the pcDNA3.1(+) vector as the starting vector to construct the recombinant plasmid.
[0059] The recombinant plasmid is shown in sequence 4 of the sequence listing. In sequence 4 of the sequence listing, the 910th-1728th nucleotide constitutes the fusion gene.
[0060] The fusion gene expresses the fusion protein shown in sequence 3 of the sequence list. In sequence 3 of the sequence listing, amino acid residues 1-33 constitute the signal peptide, amino acid residues 34-256 constitute the SARS-CoV-2 RBD, amino acid residues 257-264 constitute the Strep-tag II tag, Amino acid residues 265-272 constitute the FLAG tag.
[0061] The signal peptide in the fusion protein is recognized by the receptor on the endoplasmic reticulum membrane and combined with it, then the fusion protein reaches the lumen of the endoplasmic reticulum through the channel formed by the protein in the endoplasmic...
Embodiment 2
[0108] Example 2, the binding activity of SARS-CoV-2 RBD dimer and ACE2
[0109] Angiotensin converting enzyme 2 (angiotensin I converting enzyme, ACE2) is the receptor of SARS-CoV-2. ACE2 is shown in sequence 8 of the sequence listing.
[0110] 1. Preparation of ACE2 solution
[0111] For the preparation method of ACE2, refer to Step 2 of Example 1.
[0112] The difference lies in the replacement of the DNA molecule encoding the SARS-CoV-2 RBD with the DNA molecule encoding ACE2.
[0113] ACE2 solution is obtained.
[0114] 2. SPR test
[0115] ACE2 was coated on the CM5 chip, and then SARS-CoV-2 RBD dimer solution was added to detect the binding activity of SARS-CoV-2 RBD dimer to ACE2. see results Figure 4 .
[0116] 3. Gel filtration experiment
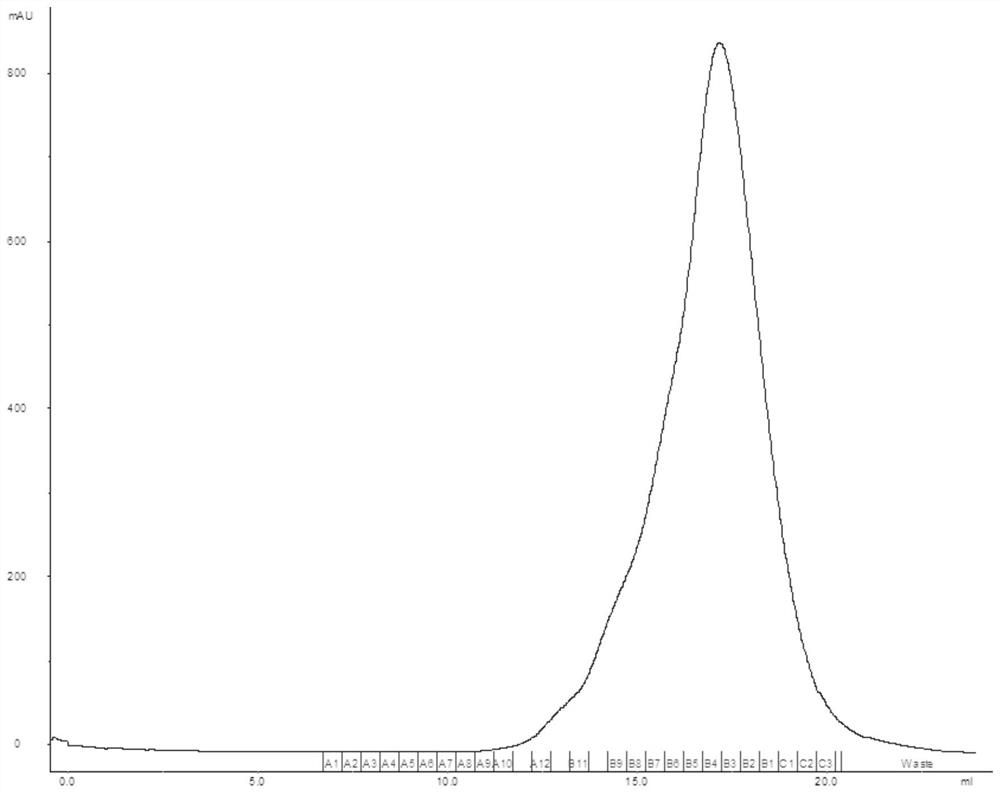
[0117] Mix 0.5ml of SARS-CoV-2 RBD dimer solution (2mg / ml protein concentration) and 0.5ml ACE2 solution (2mg / ml protein concentration) and incubate on ice for 2 hours to obtain a mixed solution (COMPLEX). The SARS-CoV-2RB...
Embodiment 3
[0119] Neutralizing activity of serum after embodiment 3, SARS-CoV-2 RBD immunization
[0120] 1. Group immunization
[0121] balB / C mice (Weitong Lihua Company) were divided into 6 groups, 5 in each group, and were immunized as follows:
[0122] The first group: the first immunization was carried out on the first day, the second immunization was carried out on the 15th day, the third immunization was carried out on the 29th day, and the fourth immunization was carried out on the 43rd day; the immunization method was intramuscular injection; The immune volume of mice is 40 μl, and the immune substance is “white emulsion formed by mixing 20 μl 1mg / ml RBD dimer solution and 20 μl Freund’s complete adjuvant”; The substance is "white emulsion formed by mixing 20 μl 1mg / ml RBD dimer solution with 20 μl Freund's incomplete adjuvant";
[0123] The second group: the first immunization was carried out on the 1st day, the second immunization was carried out on the 15th day, the third ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com