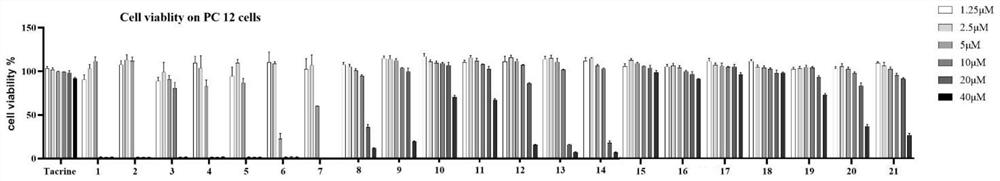
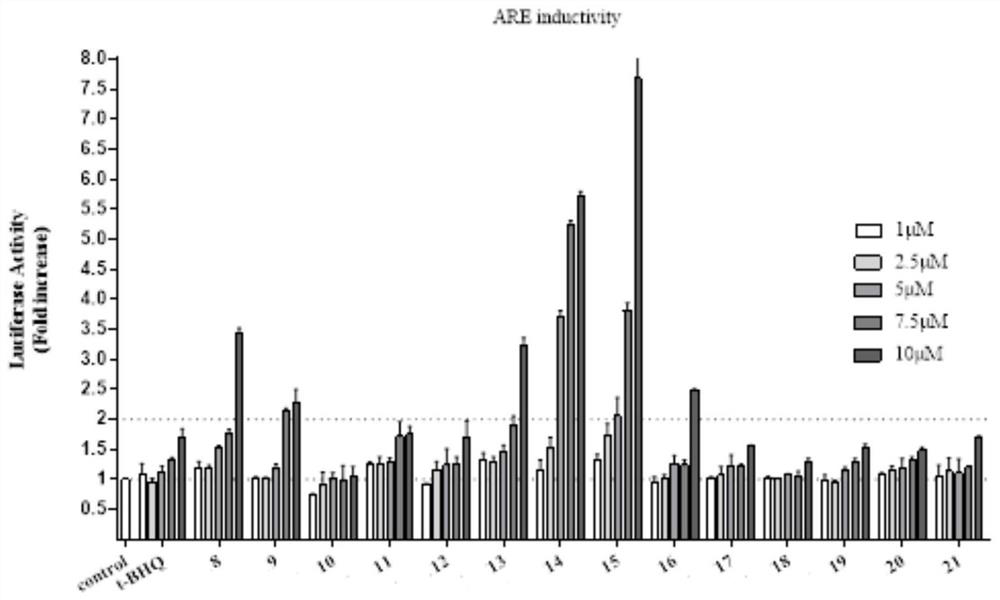
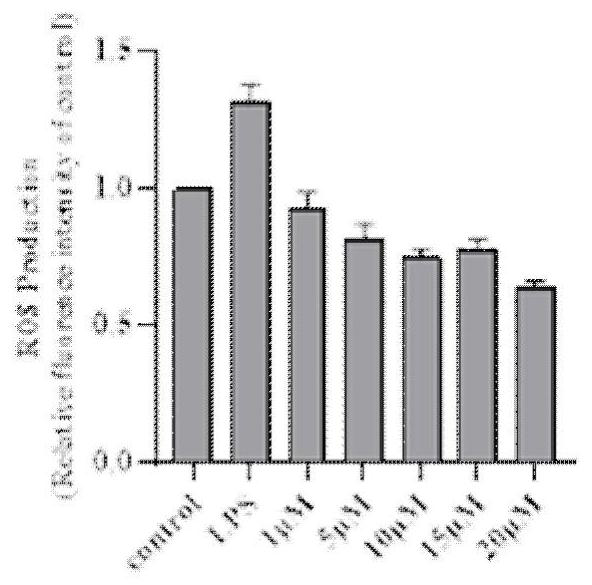
1, 2, 4-oxadiazole Nrf2 activator-tacrine spliced product as well as preparation method and application thereof
A technology of oxadiazoles and activators, applied in 1 field, can solve the problems of complex etiology and difficult to achieve treatment, and achieve the effects of high activity, small molecular weight and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthesis of 3-(3-(6-methylpyridyl))-5-(3-hydroxyphenyl)-1,2,4-oxadiazole (intermediate 1)
[0042] Dissolve 3-benzyloxybenzoic acid and N,N'-carbonyl-diimidazole in N,N'-dimethylformamide, and stir at room temperature for 45 minutes. Add 6-methyl-N-hydroxy-3-pyridinecarboxamidine into the reaction flask, then raise the temperature to 110°C and stir for 5h. The reaction solution was cooled to room temperature, poured into saturated aqueous sodium bicarbonate solution, and a white solid precipitated out. Suction filtration, the filter cake washed twice. Dried to white powder. Dissolve the white powder in ethanol, add concentrated hydrochloric acid, and reflux overnight at 105°C. After cooling to room temperature, a white solid precipitated out. Suction filtration, washed twice with water and twice with petroleum ether. After drying, a white solid was obtained. 1 H NMR (300MHz, DMSO-d6): δ10.12(s, 1H), 9.11(d, J=1.9Hz, 1H), 8.31(dd, J=8.1, 2.1Hz, 1H), 7.62(d, J ...
Embodiment 2
[0052] N-(6-(5-(5-(6-(2-methylpyridyl))1,2,4-oxadiazolyl)-2-methyl-phenoxy)hexyl)-9-amino -Synthesis of 1,2,3,4-tetrahydroacridine
[0053] With reference to the synthetic method of Example 1, intermediate 1 in Example 1 is replaced by 3-(3-(6-methylpyridyl))-5-(4-methyl-3-hydroxyphenyl)-1, 2,4-Oxadiazole, to obtain a light brown oil, which is N-(6-(5-(5-(6-(2-methylpyridyl))1,2,4-oxadiazolyl) -2-Methyl-phenoxy)hexyl)-9-amino-1,2,3,4-tetrahydroacridine. 1 H NMR (300MHz, CDCl 3 )δ9.29 (d, J=2.0Hz, 1H), 8.33 (dd, J=8.1, 2.2Hz, 1H), 7.97 (dd, J=10.6, 8.9Hz, 2H), 7.74 (dd, J=7.7 , 1.0Hz, 1H), 7.58(dd, J=14.3, 4.3Hz, 2H), 7.38(d, J=7.3Hz, 1H), 7.31(d, J=7.8Hz, 2H), 4.12(t, J =6.2Hz, 2H), 3.55(t, J=7.1Hz, 2H), 3.09(s, 2H), 2.73(s, 2H), 2.67(s, 3H), 2.31(s, 3H), 1.93(s , 6H), 1.80-1.72(m, 2H), 1.62-1.52(m, 4H). HRMS(ESI): found 548.3024, calcd for C 34 h 38 N 5 o 2 [M+H] + 548.3020.
Embodiment 3
[0055] N-(6-(5-(5-(6-(2-methylpyridyl))1,2,4-oxadiazolyl)-2-methoxy-phenoxy)hexyl)-9- Synthesis of Amino-1,2,3,4-tetrahydroacridine
[0056] With reference to the synthetic method of Example 1, intermediate 1 in Example 1 is replaced by 3-(3-(6-methylpyridyl))-5-(4-methoxy-3-hydroxyphenyl)-1 , 2,4-Oxadiazole, to obtain a light brown oil, namely N-(6-(5-(5-(6-(2-methylpyridyl)) 1,2,4-oxadiazolyl )-2-methoxy-phenoxy)hexyl)-9-amino-1,2,3,4-tetrahydroacridine. 1 H NMR (300MHz, CDCl 3 )δ9.27(s, 1H), 8.32(d, J=7.6Hz, 1H), 8.15(d, J=7.8Hz, 1H), 8.06(d, J=8.4Hz, 1H), 7.84(d, J=8.3Hz, 2H), 7.68(s, 1H), 7.65-7.58(m, 1H), 7.40(d, J=7.6Hz, 1H), 7.31(d, J=8.1Hz, 1H), 7.01( d, J=8.3Hz, 1H), 4.16(t, J=6.3Hz, 2H), 3.95(s, 3H), 3.71(t, J=7.0Hz, 2H), 3.16(s, 2H), 2.66( s, 5H), 1.91 (s, 6H), 1.86-1.77 (m, 2H), 1.59 (s, 4H). HRMS (ESI): found 564.2967, calcd for C 34 h 38 N 5 o 3 [M+H] + 564.2969.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com