2-trifluoromethyl-4-amino-quinoline derivative and application thereof
A technology of trifluoromethyl derivatives, applied in quinoline derivatives and their applications, can solve species-specific nephrotoxicity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
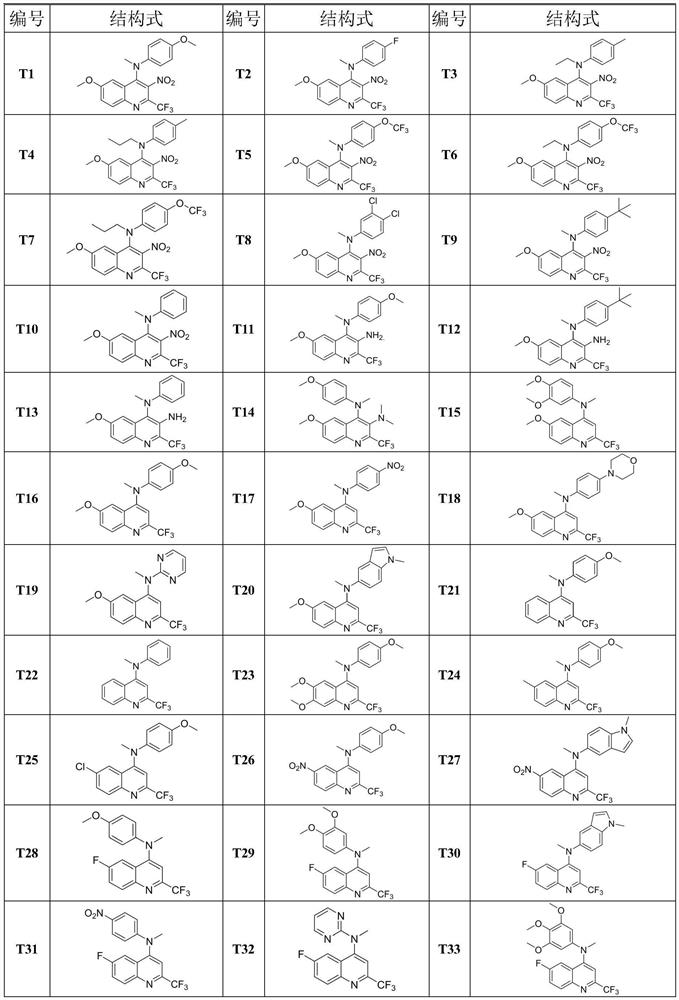
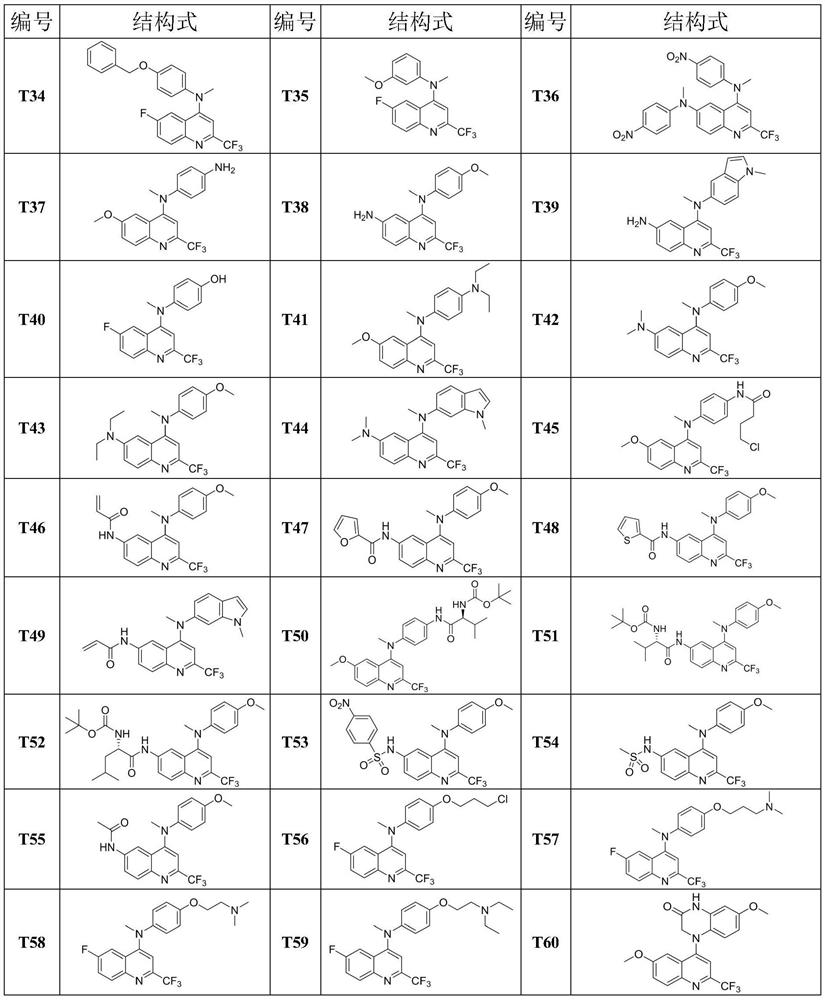
[0033] Synthesis of compound T1:
[0034] (1) Synthesis of intermediate M3a:
[0035]
[0036]Take 22.0g (64.9mmol) of polyphosphoric acid in a 250mL reaction bottle, add 2.0g (16.2mmol) of p-methoxyaniline, replace the argon, and slowly inject 2.4mL of ethyl trifluoroacetoacetate (16.3 mmol), the injection was completed and the temperature was raised to 110°C and stirred for 4 hours to complete the reaction. The reaction system was poured into ice water and stirred rapidly. It was seen that a large amount of precipitates were precipitated, filtered with suction, and the filter cake was dried under reduced pressure to obtain a total of 3.08g of compound M1a. : 78%. Take 2.00g (8.22mmol) of compound M1a, add 50mL of glacial acetic acid to dissolve it, replace the argon, and slowly inject 0.75mL (9.87mmol) of concentrated nitric acid at 0°C. A large amount of solids were precipitated in the system, which was filtered by suction, washed with water until neutral, and dried to...
Embodiment 2
[0041] Synthesis of compound T2:
[0042]
[0043] Referring to the synthesis method of T1 in Example 1, replacing 4-methoxyaniline with 4-fluoroaniline, compound T2 can be prepared as a red solid with a yield of 48.4%. 1 H NMR (600MHz, CDCl 3 )δ8.19(d,J=9.3Hz,1H),7.53(dd,J=9.3,2.8Hz,1H),6.98(d,J=2.7Hz,1H),6.97–6.91(m,2H), 6.69–6.63(m,2H),3.76(s,3H),3.39(s,3H); 13 C NMR (151MHz, CDCl 3 )δ161.06, 157.51(d, J=240.6Hz), 144.94, 144.24, 143.15, 141.82, 136.78(q, J=36.9Hz), 132.83, 129.10, 125.73, 120.25(q, J=275.9Hz), 116.40(d , J=8.1Hz), 116.11(d, J=22.5Hz), 102.09, 55.82, 39.68; 19 F NMR (565MHz, CDCl 3 ) δ-64.39.
Embodiment 3
[0045] Synthesis of Compound T3:
[0046]
[0047] Referring to the synthesis method of T1 in Example 1, replacing 4-methoxyaniline with p-toluidine and methyl iodide with ethyl iodide, the orange-red solid compound T3 can be prepared with a yield of 60.0%. 1 H NMR (600MHz, CDCl 3 )δ8.16(d, J=9.3Hz, 1H), 7.49(dd, J=9.3, 2.7Hz, 1H), 7.09–6.96(m, 3H), 6.61(d, J=8.5Hz, 2H), 3.81(q, J=7.1Hz, 2H), 3.71(s, 3H), 2.25(s, 3H), 1.31(t, J=7.1Hz, 3H); 13 C NMR (151MHz, CDCl 3 )δ 160.74, 145.00, 144.18, 143.57, 136.76 (q, J = 36.5Hz), 132.59, 130.14, 130.12, 129.98, 125.47, 119.99 (q, J = 275.8Hz), 116.13, 102.72, 55.80, 230.47. ; 19 FNMR (565MHz, CDCl 3 ) δ-64.47.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com