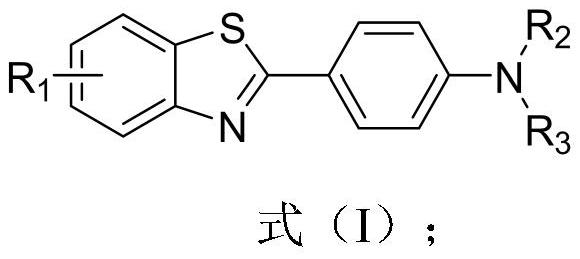
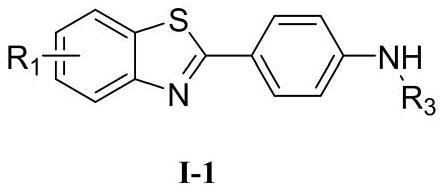
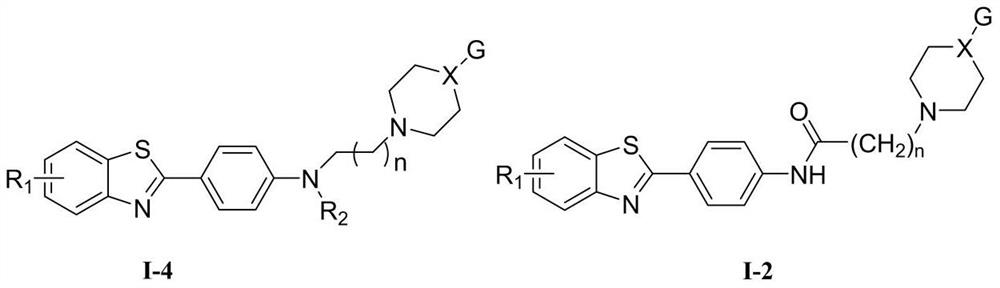
4-(benzothiazole-2-yl)-N-substituted aniline compound and preparation method and application thereof
A technology for benzothiazoles and compounds, applied in the field of 4--N-substituted aniline compounds and their preparation, to achieve novel structures, significant effects, and remarkable antiviral effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: Compound Ia (R 1 = H, R 2 = H, R 3 = cyclohexyl) synthesis
[0085] Dissolve 4-(benzothiazol-2-yl)aniline (100mg, 0.44mmol) in DMF (3mL), add sodium hydride (105mg, 4.4mmol) and bromocyclohexane (80mg, 0.49mmol), 60 Reaction at ℃ for 5h. Cool down to room temperature, add water, extract with ethyl acetate, wash with saturated brine, separate layers, dry over anhydrous sodium sulfate, filter, and remove the solvent. The obtained crude product was subjected to silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 30:1) to obtain 30 mg of white solid product Ia. 1 HNMR (400MHz, CDCl 3 )δ7.98(d, J=8.0Hz, 1H), 7.89 (d, J=8.8Hz, 2H), 7.84(d, J=7.9Hz, 1H), 7.45–7.41(m, 1H), 7.32– 7.28(m,1H), 6.62(d,J=8.9Hz,2H),3.98(d,J=6.5Hz,1H),3.37–3.34(m,1H),1.45–1.41(m, 2H),1.81 –1.76(m,2H),1.72–1.66(m,1H),1.46–1.35(m,2H),1.29–1.16(m,3H). 13 CNMR (101MHz, CDCl 3 )δ167.75, 153.34, 148.72, 133.46, 128.14, 124.92, 123.14, 121.24, 121.00, 120.30, 111....
Embodiment 2
[0086] Embodiment 2: Compound Ib (R 1 = H, R 2 = H, R 3= )Synthesis
[0087] The raw material bromocyclohexane in Example 1 was replaced by 2-(bromomethyl)-6-methoxyphenol, and the yellow solid compound Ib was obtained with reference to the method of Example 1. 1 HNMR (400MHz, DMSO-d 6 )δ8.79(s,1H), 8.00(d,J=7.4Hz,1H),7.89(d,J=8.1Hz,1H),7.78(d,J=8.4Hz,2H),7.46– 7.43( m,1H),7.35–7.31(m,1H),6.88–6.84(m,2H),6.82–6.80(m,1H),6.73–6.68(m,3H),4.30(d,J=5.9Hz, 2H),3.80(s,3H). 13 CNMR (101MHz, DMSO-d 6 )δ 168.00,153.86,151.61,147.29,143.82,133.68,128.60,126.17,125.51,124.26, 121.82,121.70,120.04,120.02,118.62,111.83,110.77.38,40.55
Embodiment 3
[0088] Embodiment 3: compound Ic (R 1 = H, R 2 = H, R 3 = )Synthesis
[0089] In embodiment 1, raw material bromocyclohexane is replaced by 3-bromomethylthiophene, and the experimental method of reference embodiment 1 obtains white solid compound Ic. 1 HNMR (400MHz, CDCl 3 )δ7.99(d, J=8.1Hz, 1H), 7.92(d, J=8.5Hz, 2H), 7.84(d, J=7.9Hz, 1H), 7.46–7.42(m, 1H), 7.36– 7.28(m, 2H), 7.21(s, 1H), 7.09(d, J=4.8Hz, 1H), 6.70(d, J=8.5Hz, 2H), 4.41(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ168.60, 154.34, 150.26, 139.54, 134.57, 129.14, 126.99, 126.49, 126.03, 124.33, 123.06, 122.40, 122.03, 121.39, 112.60, 43.31.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com