a co 4 o 4 Preparation method and application of cobalt-based metal-organic framework compound with quasi-cubane structure
A technology of organic frameworks and compounds, applied in the preparation method and application field of cobalt-based metal-organic framework catalysts, can solve the problems of unfavorable mechanism research and practical application, poor stability, etc., and achieve easy large-scale promotion and application, easy operation, and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
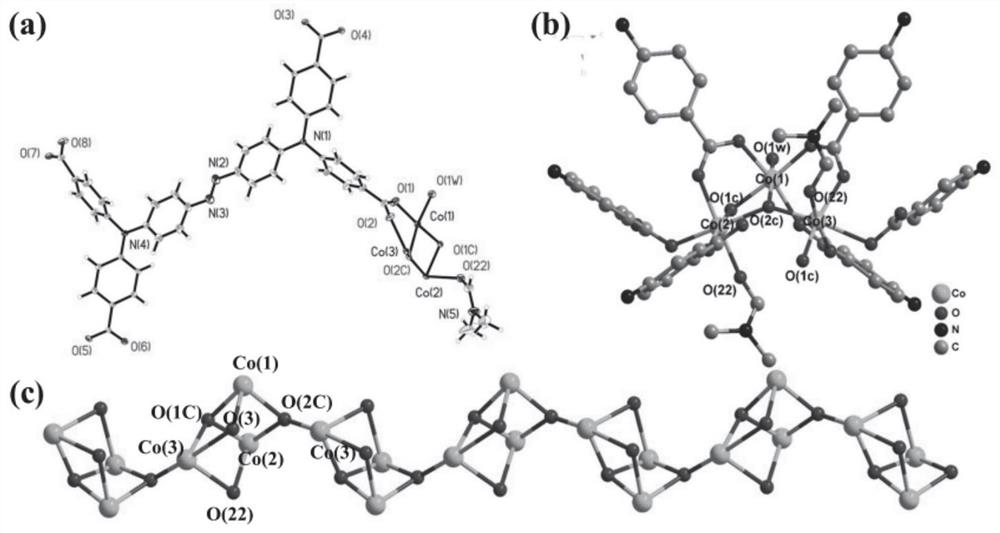
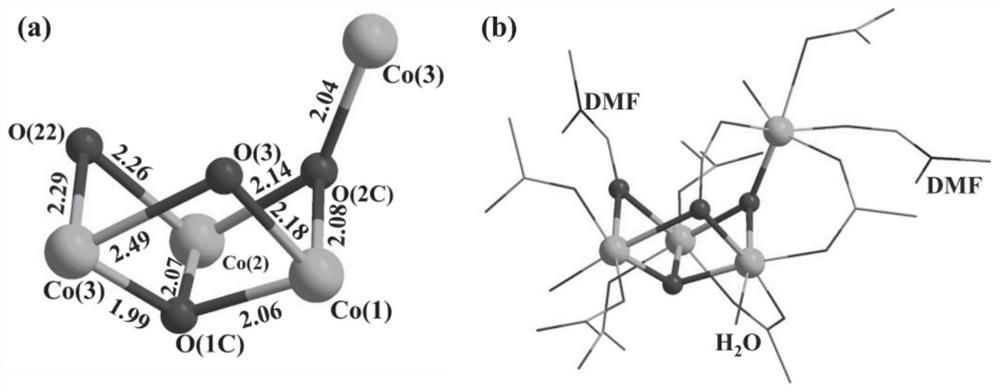
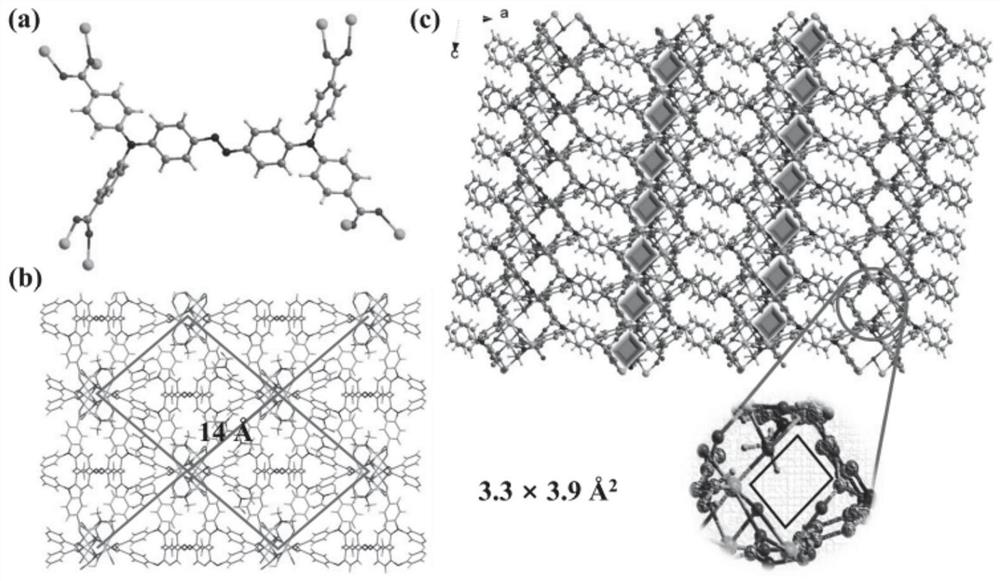
[0045] (E)-4,4',4",4"'-(N,N,N',N'-tetraphenyl-p-diaminoazobenzene in azo-linked triphenylamine carboxylic acid derivatives ) formic acid C 40 H 28 N 4 O 8 (17.3 mg, 0.025 mmol), CoCl 2 ·6H 2 O (24.0 mg, 0.10 mmol) was dissolved in N,N'-dimethylformamide (4 mL) and deionized water (2 mL) and stirred well, then the solution was taken and placed in an oven, calcined at 130 °C for 72 h, Turn off the oven, cool to room temperature, reddish-brown transparent diamond-like crystals are produced, filter, and dry to obtain the target material Co-L with a yield of about 56%. Infrared spectral peak positions (IR): 3426(w), 2930(w), 1680(s), 1647(vs), 1592(vs), 1500(m), 1392(vs), 1315(vs), 1264( m), 1179(m), 1147(s), 1101(w), 843(m), 786(s), 701(w), 560(m), 521(w), 472(w)cm -1 . The obtained target material structure is as figure 1 , figure 2 , image 3 and Figure 4 shown. Figure 1-4 The specific structure of the target material is given, from figure 1 In a, it can be see...
Embodiment 2
[0047] (E)-4,4',4",4"'-(N,N,N',N'-tetraphenyl-p-diaminoazobenzene in azo-linked triphenylamine carboxylic acid derivatives ) formic acid C 40 H 28 N 4 O 8 (17.3mg, 0.025mmol), Co(NO 3 ) 2 ·6H 2 O (30.0 mg, 0.10 mmol) was dissolved in N,N'-dimethylformamide (4 mL) and deionized water (2 mL) and stirred well, then the solution was taken and placed in an oven, calcined at 130 °C for 72 h, Turn off the oven, cool down to room temperature, reddish-brown transparent diamond-like crystals are produced, filter, and dry to obtain the target material Co-L with a yield of about 49%.
Embodiment 3
[0049] (E)-4,4',4",4"'-(N,N,N',N'-tetraphenyl-p-diaminoazobenzene in azo-linked triphenylamine carboxylic acid derivatives ) formic acid C 40 H 28 N 4 O 8 (34.6 mg, 0.05 mmol), CoCl 2 ·6H 2 O (48.0 mg, 0.20 mmol) was dissolved in N,N'-dimethylformamide (80 mL) and deionized water (40 mL) and stirred well, and then the solution was placed in a 250 mL eggplant-shaped flask at 130° C. Heating under reflux for 24 h, stopping heating and cooling to room temperature, filtering and drying to obtain an orange powder, the target material Co-L with a micron size was obtained. The scanning electron microscope (SEM) image of the obtained micron-sized target material is as follows Figure 9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com