Pharmaceutical composition containing sodium picosulfate, magnesium oxide, citric acid and/or potassium bicarbonate and preparation method thereof
A technology of sodium picosulfate and potassium bicarbonate, applied in the field of pharmaceutical preparation, can solve the problems of unavoidable risks, poor mixing uniformity, unstable products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Table 2. Composition of Embodiment 1 (unit dose packaging, every bag)
[0029] Example 1 materials weight Sodium picosulfate 10mg magnesium oxide 3.5g citric acid 12g potassium bicarbonate 0.5g sodium saccharin 60mg essence 60mg
[0030] Embodiment 1 preparation method:
[0031] Put 24kg of citric acid and 7kg of magnesium oxide into 10kg of water, heat and dissolve at 65°C to form a clear solution. Dry with spray drying equipment, the spray speed is 2.5ml / min, and the drying temperature is 140°C to obtain solid powder 1.
[0032] 1 kg of potassium bicarbonate was pulverized with a pulverizer, and added into the fluidized bed with solid powder 1, and 20 g of sodium picosulfate was dissolved in 4 kg of purified water. The sodium picosulfate solution is atomized and sprayed into the fluidized bed to granulate the materials in the fluidized bed. Dry until the material temperature is 40° C., use a 0.6 mm sieve...
Embodiment 2-9
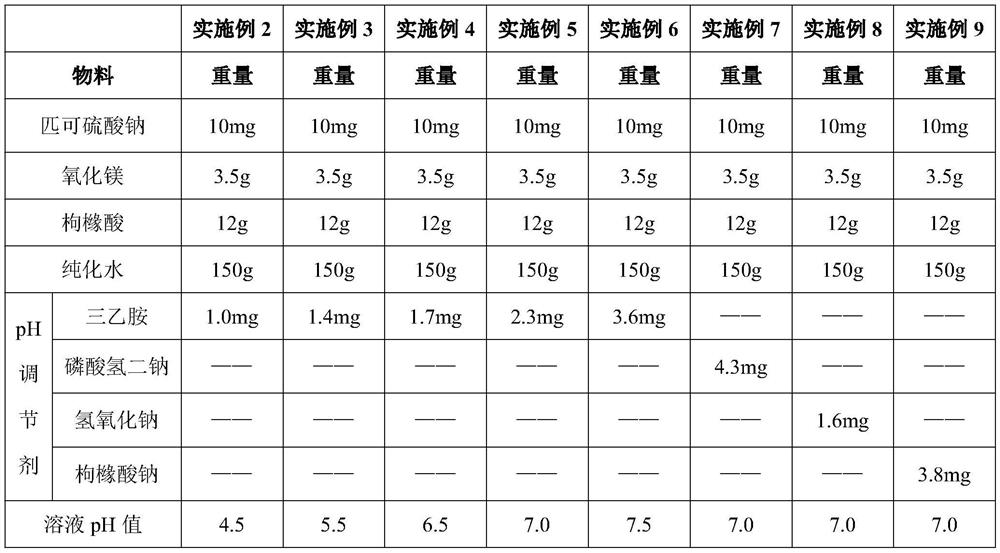
[0038] Table 3. The concrete composition of embodiment 2-9
[0039]
[0040] The preparation method of embodiment 2-9:
[0041]Take 12 g of citric acid and put it into 150 g of purified water, stir and dissolve, then add 3.5 g of magnesium oxide, add a pH regulator, and finally add 10 mg of sodium picosulfate to obtain a mixed solution.
[0042] Investigation on the stability of sodium picosulfate content
[0043] Measure the solution pH and content change of embodiment 2-9 and see the following table:
[0044] Sodium picosulfate content stability investigation table
[0045] sample 0 January March Example 2 93.3 89.1 79.6 Example 3 94.6 91.5 85.9 Example 4 98.5 97.6 97.5 Example 5 98.9 98.6 98.5 Example 6 98.7 98.1 97.9 Example 7 86.3 85.3 84.7 Example 8 90.1 89.7 89.4 Example 9 95.4 93.1 91.8
[0046] By using different pH regulators to adjust to different pHs, the content of sodium pico...
Embodiment 10-13
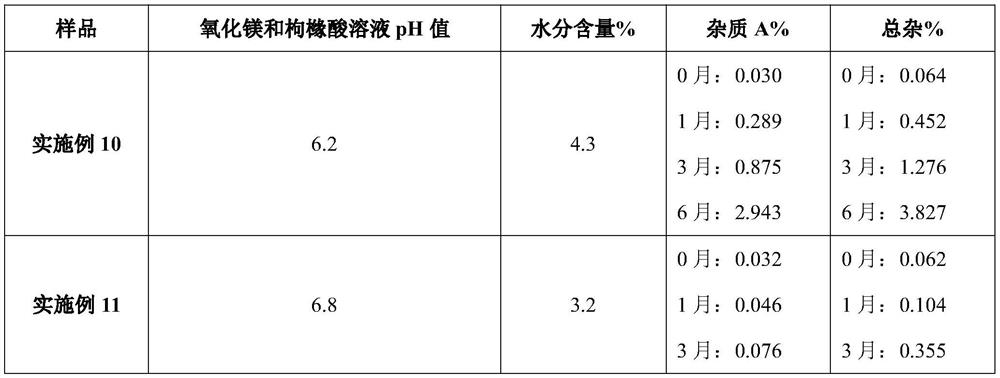
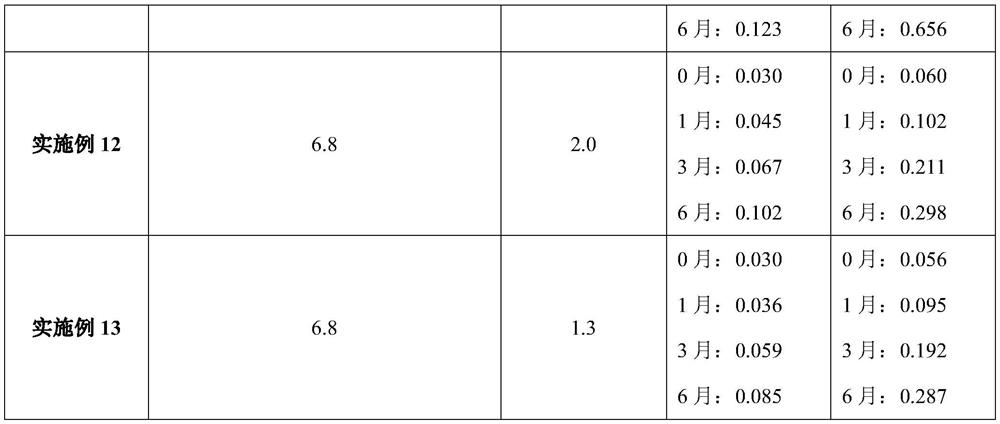
[0048] Table 4. Compositions of Examples 10-13 (unit dose packaging, per bag)
[0049] Example 10 Example 11 Example 12 Example 13 materials weight weight weight weight Sodium picosulfate 10mg 10mg 10mg 10mg magnesium oxide 3.5g 3.5g 3.5g 3.5g citric acid 12g 12g 12g 12g potassium bicarbonate 0.5g 0.5g 0.5g 0.5g sodium saccharin 60mg 60mg 60mg 60mg essence 60mg 60mg 60mg 60mg Triethylamine 2.5mg 2.5mg 2.5mg 2.5mg Dry material temperature °C 35 40 45 50
[0050] Embodiment 10-13 preparation method:
[0051] Put 24kg of citric acid, 7kg of magnesium oxide and 5g of triethylamine into 10kg of water, heat and dissolve at 65°C to form a clear solution. Dry with spray drying equipment, the spray speed is 2.5ml / min, and the drying temperature is 140°C to obtain solid powder 1.
[0052] 1 kg of potassium bicarbonate was pulverized with a pulverizer, and added int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com