Mn5O8 nano cage-shaped oxygen reduction electrocatalyst and preparation method thereof
An electrocatalyst and nanocage technology, which is applied in the field of Mn5O8 nanocage oxygen reduction electrocatalyst and its preparation, can solve the problems that the catalytic activity of non-precious metal catalysts cannot meet the requirements, the amount of precious metal Pt is high, and the commercial cost is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
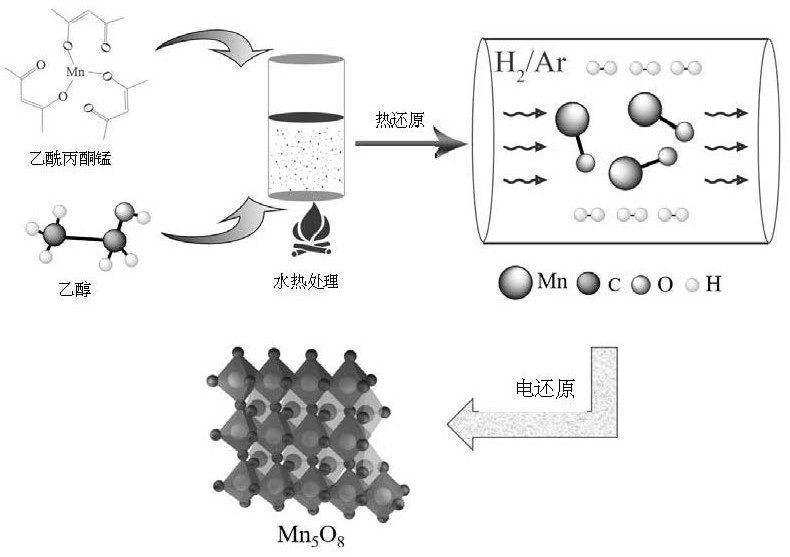
[0031] mn 3 o 4 Preparation: Dissolve 0.5-2 mmol of manganese acetylacetonate in 20-80 mL of alcohol solution, then transfer the solution to a hydrothermal kettle, react at 100-120°C for 10-14 hours, and then centrifuge the resulting mixture with alcohol for three Centrifuge once with water, and finally collect by freeze-drying for 10-24 hours. The hydrothermal reaction can also be carried out under high pressure, and the pressure can be 2-20 MPa.
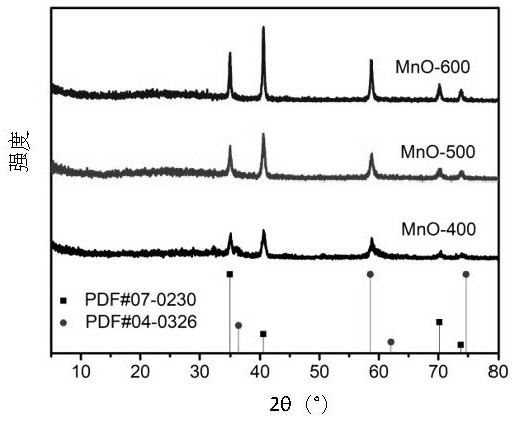
[0032] Preparation of MnO: React the above obtained sample at 400-700° C. for 1-4 hours in a reducing atmosphere. The reducing atmosphere is 5% H 2 and 95% Ar mixture or 10% H 2 and 90% Ar mixture. above H 2 Or the percentage of Ar refers to the volume ratio. The heating rate may be 2-5° C. / min.
[0033] mn 5 o 8 Preparation: Take 5 mg of the above-mentioned MnO nanoflower powder and 5 mg of carbon black in a 5 mL centrifuge tube, add 90-200 μL of deionized water and 770-900 μL of isopropanol at the same time, sonicate fo...
Embodiment 1
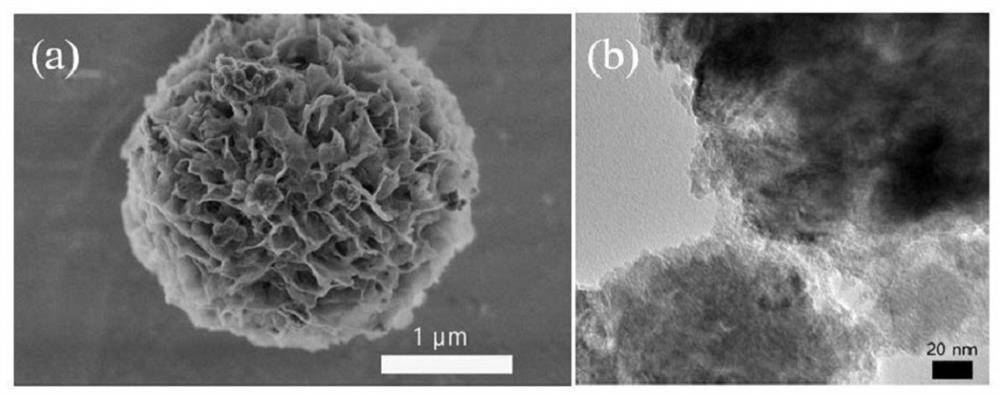
[0038] According to above-mentioned technical scheme and process flow of the present invention, at first prepare Mn 3 o 4 Nanoflower precursor materials. Weigh 1mmol of manganese acetylacetonate and dissolve it in 40mL of alcohol solution, then transfer the solution to a hydrothermal kettle, react at 120°C for 10h, then centrifuge the resulting mixture three times with alcohol, once with water, and finally through freezing Collected after drying for 12h to obtain Mn 3 o 4 Nanoflower precursor materials.
[0039] The above obtained Mn 3 o 4 Nanoflower precursor material in 5% H 2 and 95% Ar mixed gas at 400° C. for 2 h with a heating rate of 5° C. / min to obtain MnO nanoflower powder.
[0040] Next is Mn 5 o 8 Catalyst preparation: Take 5 mg of the above MnO nanoflower powder and 5 mg of carbon black in a 5 mL centrifuge tube, add 190 μL of deionized water and 800 μL of isopropanol at the same time, sonicate for 0.5 hours, then add 10 μL of Nafion solution, and sonicate...
Embodiment 2
[0043] According to process flow described (with embodiment 1), at first prepare Mn 3 o 4 Nanoflower precursor materials. Then the above obtained Mn 3 o 4 Nanoflower precursor material in 5% H 2 react with a mixed gas of 95% Ar at 500° C. for 2 hours, and the heating rate is 5° C. / min to obtain MnO nanoflower powder.
[0044] Next is Mn 5 o 8 Catalyst preparation: Take 5 mg of the above MnO nanoflower powder and 5 mg of carbon black in a 5 mL centrifuge tube, add 120 μL of deionized water and 860 μL of isopropanol at the same time, sonicate for 0.5 hours, then add 10 μL of Nafion solution, and sonicate again for 0.5 hours to obtain Suspension with a concentration of 5 mg / mL;
[0045] Take the above suspension and apply it on the surface of the glassy carbon electrode, use the glassy carbon electrode as the working electrode, the graphite rod as the counter electrode, and Ag / AgCl as the reference electrode, connect it correctly in the electrolytic cell and use the chrono...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com