Cu-doped double perovskite material and preparation method thereof
A double perovskite and precursor solution technology, which is applied in chemical instruments and methods, bismuth compounds, inorganic chemistry, etc., can solve the problems of weak light absorption ability and limited application, so as to improve light absorption and light absorption intensity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) CsBr, AgBr, BiBr 3 、CuBr 2 Mix according to the molar ratio of 2.00:0.95:0.95:0.10, add to 15mL of hydrobromic acid, heat and stir at 110°C for about 2 hours, and obtain a precursor solution with a concentration of 0.07M;
[0031] (2) The precursor solution was quickly transferred to a polytetrafluoroethylene reactor, and heated at 150°C for 24 hours;
[0032] (3) The reactor was taken out, and cooled naturally at room temperature. The reaction solution was taken out and filtered. The filtered part was washed with water and organic solvent respectively, and then dried in vacuum to obtain Cs 2 (Ag 0.95 Bi 0.95 Cu 0.10 )Br 6 crystals.
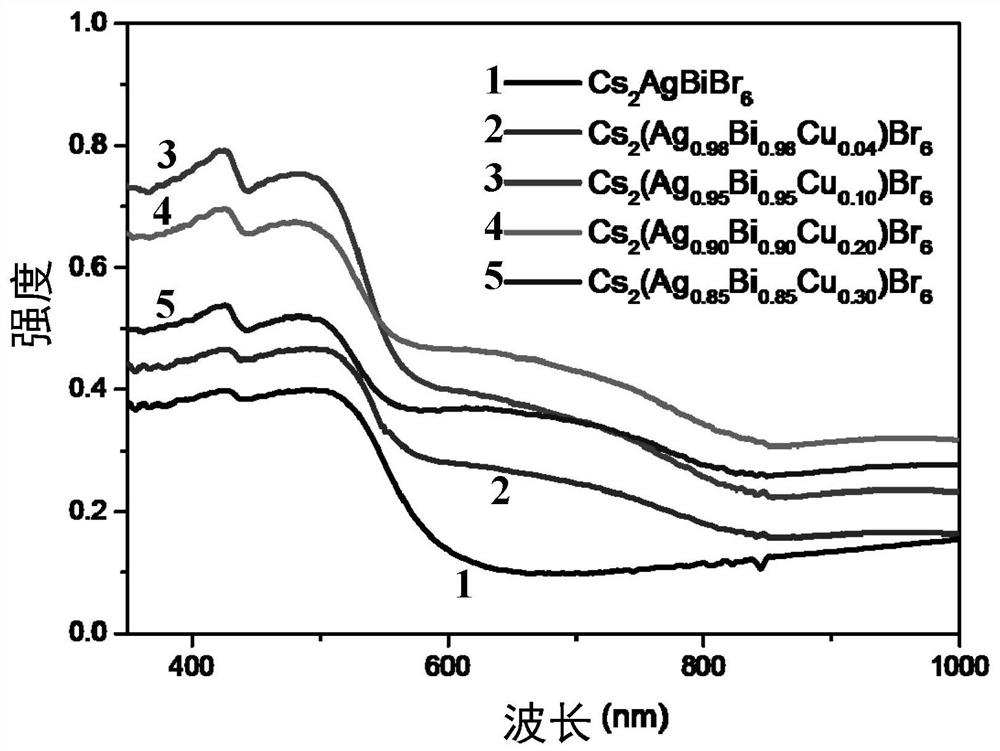
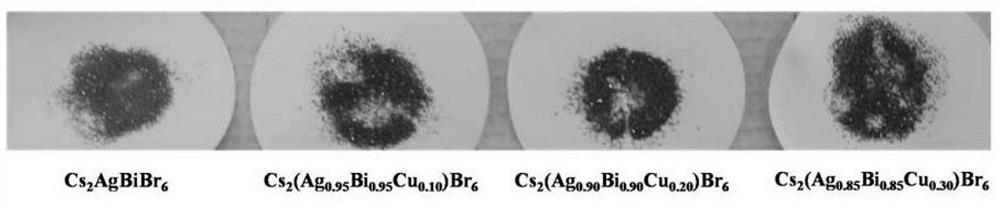
[0033] Cs obtained in the present embodiment 1 2 (Ag 0.95 Bi 0.95 Cu 0.10 )Br 6 The physical picture of the crystal is as figure 1 Shown, comparative example Cs 2 AgBr 6 For orange-red crystals, and Cs 2 (Ag 0.95 Bi 0.95 Cu 0.10 )Br 6 For dark brown crystals.
[0034] With present embodiment 1 gained Cs 2 (Ag 0....
Embodiment 2
[0038] (1) CsBr, AgBr, BiBr 3 、CuBr 2 Mix according to the molar ratio of 2.00:0.95:0.95:0.10, add to 20mL hydrobromic acid, heat and stir at 110°C for about 2 hours, and obtain a precursor solution with a concentration of 0.05M;
[0039] (2) quickly transfer the precursor solution to a polytetrafluoroethylene reactor, and heat and keep it at 170°C for 24h;
[0040] (3) The reactor was taken out, and cooled naturally at room temperature. The reaction solution was taken out and filtered. The filtered part was washed with water and organic solvent respectively, and then dried in vacuum to obtain Cs 2 (Ag 0.95 Bi 0.95 Cu 0.10 )Br 6 crystals.
[0041] Gained Cs in this embodiment 2 2 (Ag 0.95 Bi0 .95 Cu 0.10 )Br 6 Crystal size, performance are similar to Example 1.
Embodiment 3
[0043] (1) CsBr, AgBr, BiBr 3 、CuBr 2 Mix according to the molar ratio of 2.00:0.90:0.90:0.20, add to 20mL hydrobromic acid, heat and stir at 115°C for about 3 hours, and obtain a precursor solution with a concentration of 0.07M;
[0044] (2) The precursor solution is quickly transferred to a polytetrafluoroethylene reactor, and heated at 150° C. for 36 hours;
[0045] (3) The reactor was taken out, and cooled naturally at room temperature. The reaction solution was taken out and filtered. The filtered part was washed with water and organic solvent respectively, and then dried in vacuum to obtain Cs 2 (Ag 0.90 Bi 0.90 Cu 0.20 )Br 6 crystals.
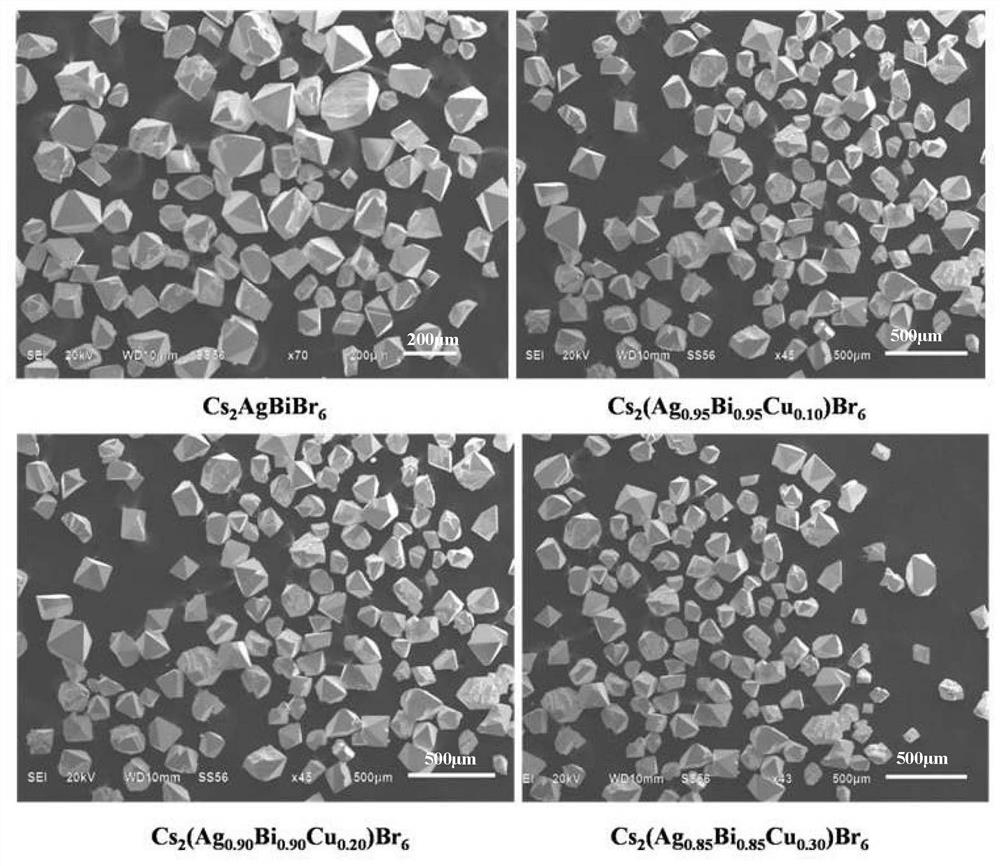
[0046] The Cs obtained in Example 3 2 (Ag 0.90 Bi 0.90 Cu 0.20 )Br 6 The grain size distribution of the crystal is 50-200 μm (see figure 2 ), the crystal structure remains Cs 2 AgBr 6 The cubic phase structure of the perovskite phase (see image 3 ). Light absorption intensity and comparative example 1Cs 2 AgBr 6 Com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com