Compound as potassium channel modulator and their preparation and use
A technology of compounds and uses, applied in the field of biomedicine, can solve problems such as poor selectivity and adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
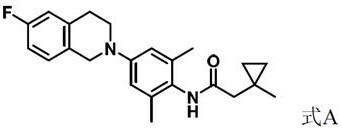
[0060] The preparation of embodiment 1 compound A
[0061]
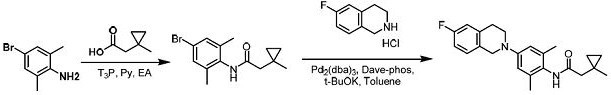
[0062] Step 1, compound 2
[0063] Compound 1 (2.0 g, 10.0 mmol, 1.0 eq) was dissolved in ethyl acetate (100 mL), and 2-(1-methylcyclopropyl)acetic acid (cas:71199-15-0, 1.26 g, 11.0 mmol , 1.1 eq), pyridine (7.9 g, 99.96mmol, 10.0 eq) and T 3 P (50%, 31.8 g, 49.97 mmol, 5.0 eq), heated to 50°C and stirred for 16 hours. After cooling to 25 ºC, dilute with water and extract with ethyl acetate (3 x 100 mL). The combined organic phases were washed with saturated sodium chloride solution and dried over anhydrous sodium sulfate, and the concentrated residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1) to obtain compound 2 (2.5 g, 84 %) as a white solid.
[0064] LCMS: [M+H] + = 296.0
[0065] Step 2, Compound A
[0066] Compound 3 (224mg, 0.61mmol, 1.2eq) was dissolved in toluene (5 mL), followed by adding compound 2 (150mg, 0.51mmol, 1.0 eq), potassium tert-butoxide (172...
Embodiment 2
[0069] Example 2 Potassium channel opener agonist rate test (FDSS / μCELL detection)
[0070] 1. Experimental method:
[0071] 1.1 Experimental process
[0072] Cell preparation: CHO-KCNQ2 cells were cultured at 175 cm 2 In the culture flask, when the cell density grows to 60-80%, remove the culture medium, wash with 7 mL PBS (Phosphate Buffered Saline, phosphate buffer saline), and then add 3 mL 0.25% Trypsin for digestion. After the digestion is complete, add 7 mL of culture medium (90% DMEM / F12 + 10% FBS + 500 μg / mL G418) to neutralize, centrifuge at 800 rpm for 3 minutes, suck off the supernatant, and then add 5 mL of culture medium to resuspend the cells. count.
[0073] Cell plating: Adjust the density to 3x10 according to the cell count results 4 per well, after standing at room temperature for 30 minutes, place in 37°C CO 2 Incubate overnight in an incubator for 16-18 hours to reach a cell density of approximately 80%.
[0074] Fluorescent dye incubation: Discard t...
Embodiment 3
[0101] The research of embodiment 3 compound through blood-brain barrier ability
[0102] 1) Research purpose: In order to obtain the situation of the tested compound passing through the blood-brain barrier
[0103] 2) Experimental content
[0104] Take 9 healthy male ICR mice (body weight range: 18-22 grams), divide them into 3 groups, 3 mice / group, orally administer the compound to be tested after fasting overnight, and undergo cardiac puncture at time points 1h, 2h and 4h Blood collection, collect at least 0.5 mL whole blood to EDTA-K 2 Centrifuge the anticoagulant tube within half an hour to collect plasma (6000 rpm, 8 minutes, 4°C), and freeze it at -20°C for later use. At the same time, the brain tissue was collected, rinsed with normal saline, blotted dry with absorbent paper, weighed, and frozen at -20°C for later use.
[0105] Experimental results: According to the obtained blood drug concentration data, the pharmacokinetic parameters after administration were calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com