Compound astragalus polysaccharide soluble powder for preventing and treating avian influenza
An astragalus polysaccharide, soluble technology, applied in powder delivery, pharmaceutical formulations, allergic diseases and other directions, to achieve the effects of abundant raw materials, low price and good use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
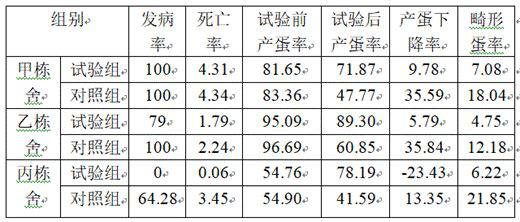
Image
Examples
Embodiment 1
[0017] When the present invention is implemented, the following weights can be used: 550 g of astragalus polysaccharide, 180 g of Achyranthes bidentata polysaccharide, 110 g of cat's claw polysaccharide and 75 g of semen japonicus cream are used as raw material drugs, and the above-mentioned raw materials are mixed and dried, and the weight of the raw material drugs is added. 3% auxiliary material, the auxiliary material is powdered sugar and dextrin mixed at a ratio of 4:1, thoroughly mixed, pulverized, dried at 60°C, and passed through an 80-mesh sieve to obtain the compound astragalus polysaccharide soluble powder.
Embodiment 2
[0019] When the present invention is implemented, the following weights can be used: 600g of astragalus polysaccharide, 200g of Achyranthes bidentata polysaccharide, 120g of cat's claw polysaccharide and 80g of semen japonicus cream as the raw material drug, the above-mentioned raw materials are mixed and dried, and the weight of the raw material drug is added 3% auxiliary material, the auxiliary material is powdered sugar and dextrin mixed at a ratio of 4:1, thoroughly mixed, pulverized, dried at 60°C, and passed through an 80-mesh sieve to obtain the compound astragalus polysaccharide soluble powder.
Embodiment 3
[0021] When the present invention is implemented, the following weights can also be used: 650g of astragalus polysaccharide, 220g of Achyranthes bidentata polysaccharide, 130g of cat's claw polysaccharide and 85g of semen japonicus cream as the raw material drug, the above-mentioned raw materials are mixed and dried, and the raw material drug is added 3% by weight of auxiliary materials, the auxiliary materials are powdered sugar and dextrin, fully mixed at a ratio of 4:1, pulverized, dried at 60°C, and passed through an 80-mesh sieve to obtain the compound astragalus polysaccharide soluble powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com