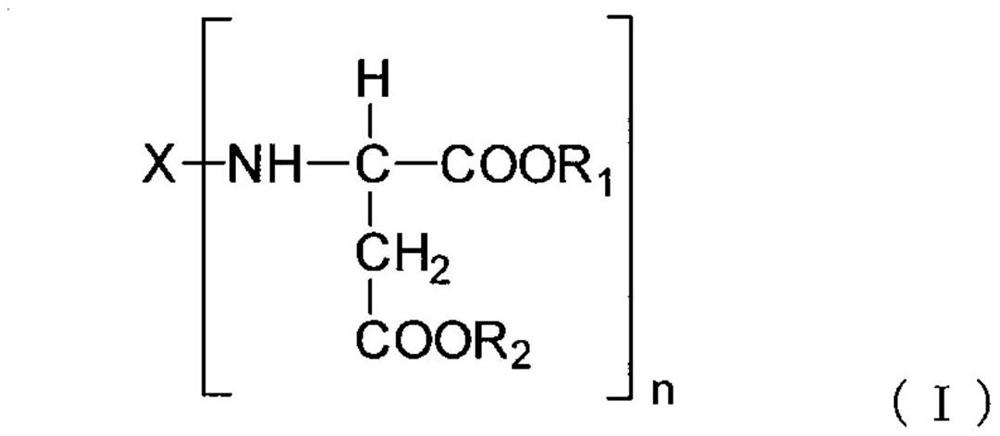
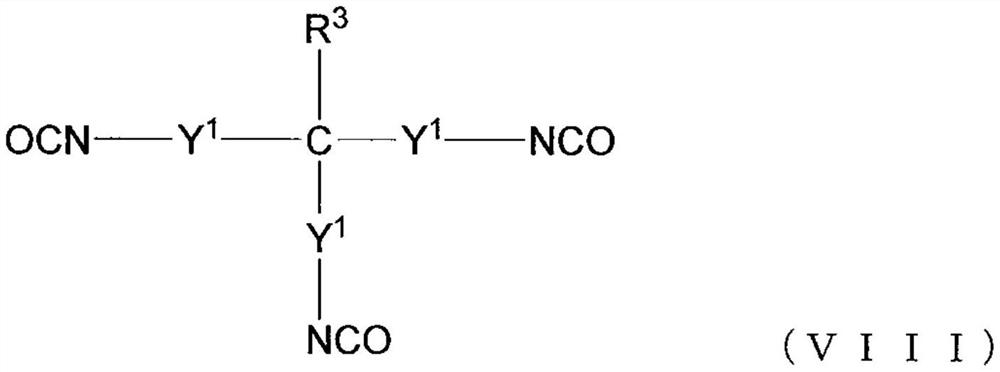
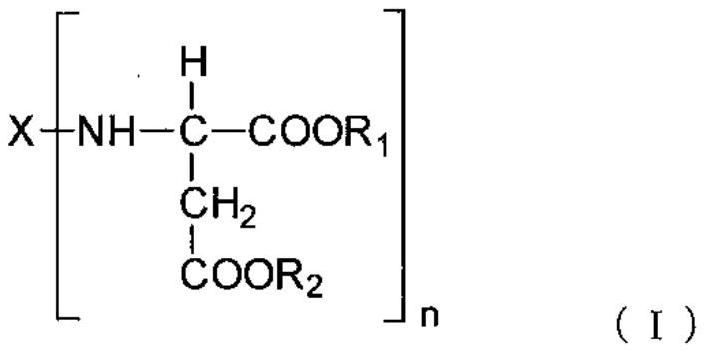
Polyaspartic acid coating composition, coating film, and coated article
A technology of polyaspartic acid and aspartic acid ester, which is applied in polyurea/polyurethane coatings, synthetic resin layered products, and devices for coating liquid on the surface, etc., to achieve excellent pot life, excellent hardness and weather resistance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0315] Hereinafter, although an Example demonstrates this embodiment in more detail, this embodiment is not limited to these Examples. The measurement of various physical properties and the method of various evaluation are demonstrated below. In addition, unless otherwise indicated, "part" and "%" mean "part by mass" and "% by mass".
[0316] (Physical properties 1) NCO content (mass%)
[0317] The NCO content (isocyanate content, mass %) of the polyisocyanate was measured as follows. 1 to 3 g of the polyisocyanate produced in the production example was accurately weighed (Wg) in a Erlenmeyer flask, and 20 mL of toluene was added to completely dissolve the polyisocyanate. Thereafter, 10 mL of a toluene solution of 2 equivalents of di-n-butylamine was added, mixed completely, and then left to stand at room temperature for 15 minutes. Furthermore, 70 mL of isopropanol was added to this solution, and it mixed completely. Titrate the solution with 1 N hydrochloric acid solutio...
manufacture example 1-1
[0417] A 4-necked flask equipped with a stirrer, a thermometer, a reflux condenser, a nitrogen blowing tube, and a dropping funnel was made into a nitrogen atmosphere, and 100 parts of HDI were put into it, and the temperature in the reactor was kept at 60° C. while stirring. Add 0.15 parts of a solution obtained by diluting tetrabutylammonium acetate, an isocyanuration reaction catalyst, to 10% by mass with 2-ethyl-1-hexanol to carry out isocyanuration reaction. When the NCO content of the liquid became 43.8% by mass, phosphoric acid was added to stop the reaction. Thereafter, the reaction solution was kept at 90° C. for 1 hour. After the cooled reaction solution was filtered, the unreacted HDI was removed with a thin film evaporation tank. Polyisocyanate P-1 with an NCO content of 23.1% by mass, a viscosity at 25°C of 1350 mPa.s, a number average molecular weight of 590, an average number of isocyanate groups of 3.2 and a mass concentration of HDI monomer of 0.1% by mass wa...
manufacture example 1-2
[0419] Make the 4-necked flask equipped with a stirrer, a thermometer, a reflux condenser, a nitrogen blowing pipe, and a dropping funnel a nitrogen atmosphere, put in 100 parts of HDI and 0.12 parts of isobutanol, and keep the temperature in the reactor at 80°C for 2 Hour. 0.08 parts of a solution obtained by diluting the isocyanuration reaction catalyst trimethyl-2-methyl-2-hydroxyethylammonium hydroxide to 5% by mass with isobutanol was added thereto to carry out isocyanuric acid For the esterification reaction, phosphoric acid was added when the conversion rate became 20% to stop the reaction. Thereafter, the reaction liquid was kept at 160° C. for 1 hour. After the cooled reaction solution was filtered, the unreacted HDI was removed with a thin film evaporation tank. Polyisocyanate P-2 with an NCO content of 23.2% by mass, a viscosity at 25°C of 470 mPa.s, a number average molecular weight of 540, an average number of isocyanate groups of 3.0, and a mass concentration o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com