Method for preparing 2, 5-dimethyloltetrahydrofuran through hydrogenation of 5-hydroxymethylfurfural
A 5-HMF, hydrogen technology, applied in chemical instruments and methods, catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of complex reaction process, increased cost, etc., and achieve simple operation. , The effect of low equipment requirements and high product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Catalyst preparation: weigh 0.003mol of Ni(NO 3 ) 2 ·6H 2 O and 0.009mol of Co(NO 3 ) 2 ·6H 2 O in 150ml deionized water, then add 1g of CeO 2 to obtain precursors. Then weigh 3g Na 2 CO 3 and 1g NaOH in 10ml deionized water. Add dropwise to the precursor mixture and keep stirring. Stir until pH = 10, continue to age for 10h. The aged mixture was washed to neutral with deionized water. After that, it was dried in an oven at 100°C for 12 hours. Then calcined at 500°C for 4h, and reduced in hydrogen atmosphere before use, the bimetallic catalyst can be obtained.
[0016] Preparation of BHMTHF: Weigh 0.2g 5-HMF, 10mL water and 0.06g catalyst (Ni x -Co y / CeO 2 , where x=1, y=3, CeO 2 as a carrier) and put it into a 40mL reactor. Replace the air in the kettle with nitrogen for three to four times, then fill it with hydrogen until the initial pressure is 3MPa, turn on the stirring device at about 400r / min, heat to 110°C and react for 4h, then cool down, the ...
Embodiment 2
[0018] The preparation method of catalyst is the same as embodiment 1, but wherein CeO 2 Replaced by gas phase SiO 2 , and the reacted catalyst is recovered by a magnet for repeated cycle experiments.
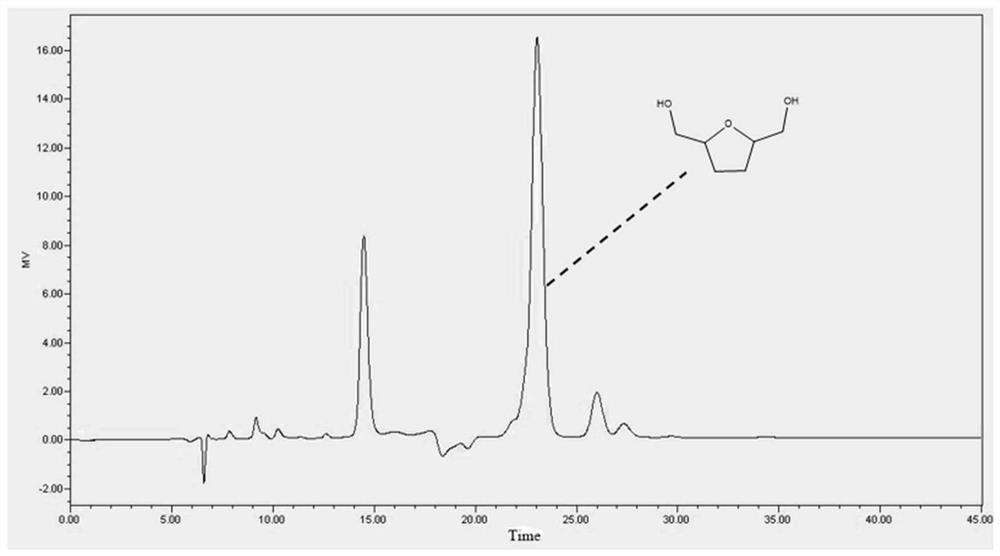
[0019] The preparation method of BHMTHF was carried out with reference to the method in Example 1. Product adopts high performance liquid chromatography (HPLC, Thermo Scientific) to carry out qualitative and quantitative analysis, the liquid phase chromatogram under this condition is as follows image 3 shown. The peak at 22-24min represents BHMTHF. As a result, the conversion rate of 5-HMF was 100%, and the yield rate of BHMTHF was 82.9%.
Embodiment 3-5
[0021] Prepare by the method for embodiment 2 and obtain corresponding bimetallic metal catalyst, wherein the Co / Ni atomic ratio in embodiment 3 is 1 / 1 (promptly take the Ni (NO of 0.003mol) 3 ) 2 ·6H 2 O and 0.003mol of Co(NO 3 ) 2 ·6H 2 O); Co / Ni atomic ratio in embodiment 4 is 2 / 1 (promptly taking the Ni (NO of 0.003mol 3 ) 2 ·6H 2 O and 0.006mol of Co(NO 3 ) 2 ·6H 2 O); the Co / Ni atomic ratio in embodiment 5 is 4 / 1 (the Ni of 0.003mol (NO 3 ) 2 ·6H 2 O and 0.012mol of Co(NO 3 ) 2 ·6H 2 O).
[0022] The catalyst prepared above was tested according to the method of Example 2, and the conversion rate and selectivity of the product obtained by the reaction were measured, which are listed in Table 1.
[0023] Table 1 Preparation of different types of catalysts and their effects on the conversion and selectivity of 5-HMF aqueous phase hydrogenation to BHMTHF
[0024]
[0025] Comprehensive embodiment 1 to 5 know, carrier gas phase SiO 2 Compared with CeO 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com