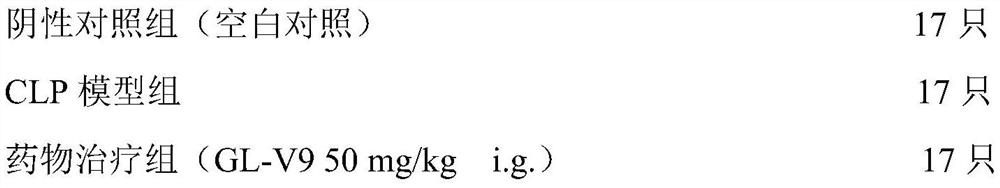Application of GL-V9 in preparation of medicine for preventing and/or treating sepsis
A technology of GL-V9 and sepsis, which is applied in the direction of pharmaceutical formulations, antibacterial drugs, medical preparations containing active ingredients, etc., can solve the problems of tissue damage and damage to normal physiological functions of the body, and reduce the release of inflammatory factors , restore macrophage viability, and inhibit cytotoxic damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 GL-V9 can reduce the degree of pyroptosis and significantly restore the macrophage J774A.1 induced by liposome 2000 transfection of lipopolysaccharide LPS into macrophages and Escherichia coli infection of macrophages Decreased cell viability and increased cytotoxic damage.
[0029] 1. Experimental materials
[0030] (1) Drugs
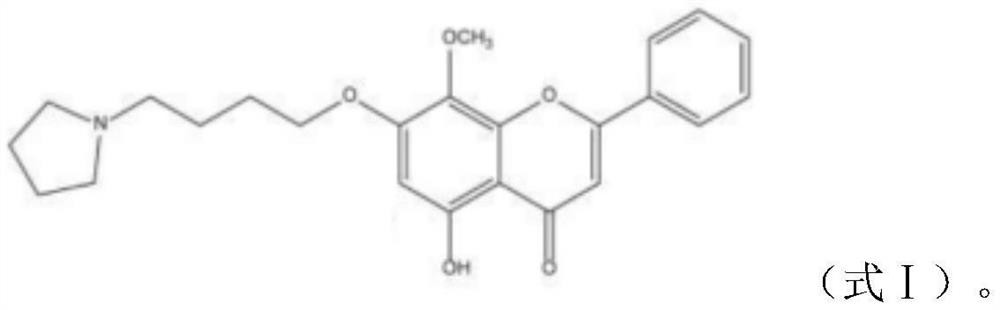
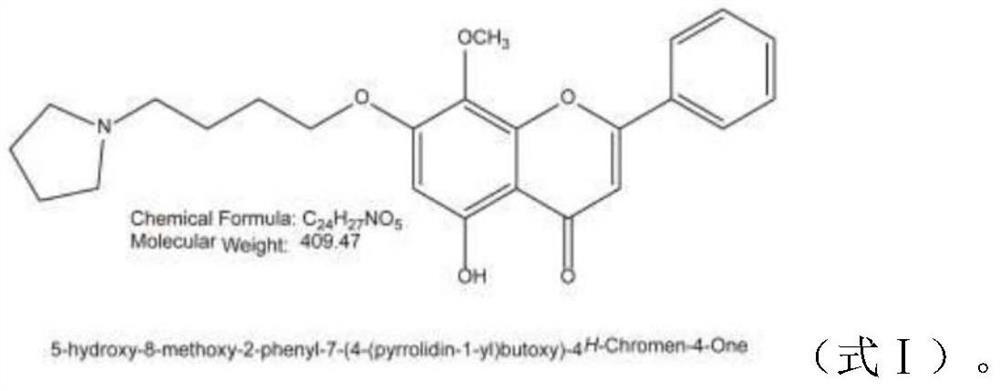
[0031] GL-V9(C 24 h 27 o 5 N, molecular weight: 409.47) provided by China Pharmaceutical University, light yellow powder with a purity of 98%, prepared into a mother liquor with DMSO, and stored at -20°C. Dilute to the required working concentration before use.
[0032] (2) cell line
[0033] The mouse mononuclear macrophage cell line J774A.1 was purchased from the cell bank of the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, and cultured in DMEM medium containing 10% fetal bovine serum.
[0034] (3) Reagents
[0035] 1) Culture medium: DMEM medium was purch...
Embodiment 2
[0089] Example 2 GL-V9 can reduce the mortality rate of cecal ligation and puncture (CLP) model mice, improve organ function damage and inhibit the release of inflammatory factors.
[0090] 1. Experimental materials
[0091] (1) Drugs:
[0092] GL-V9 is provided by Jiangsu Provincial Key Laboratory of Tumor Occurrence and Intervention, Jiangsu Pharmaceutical University. The purity is over 99%. Transparent yellow liquid for intraperitoneal injection.
[0093] (2) Reagents
[0094] 1) ELISA kits for mouse IL-1β, IL-6, IL-18 and TNF-α: Wuhan Aibotec Biotechnology Co., Ltd.
[0095] (3) Experimental animals
[0096] Source, species, and strain: C57BL / 6J mice, provided by Changzhou Cavens Experimental Animal Co., Ltd. (Experimental animal production license: SCXK (Su) 2016-0011).
[0097] Age: 6-8 weeks
[0098] Weight: 18-22g
[0099] Gender: Male
[0100] 2. Experimental method:
[0101] (1) Grouping of experimental animals
[0102]
[0103] (2) Cecal ligation and pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com