Application of exogenous recombinant protein reelin in the preparation of drugs for the treatment of cerebral hemorrhage
A recombinant protein, exogenous technology, applied in the field of biomedicine, can solve problems such as unreported effects, and achieve the effect of reducing pyroptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Establishment of intracerebral hemorrhage model
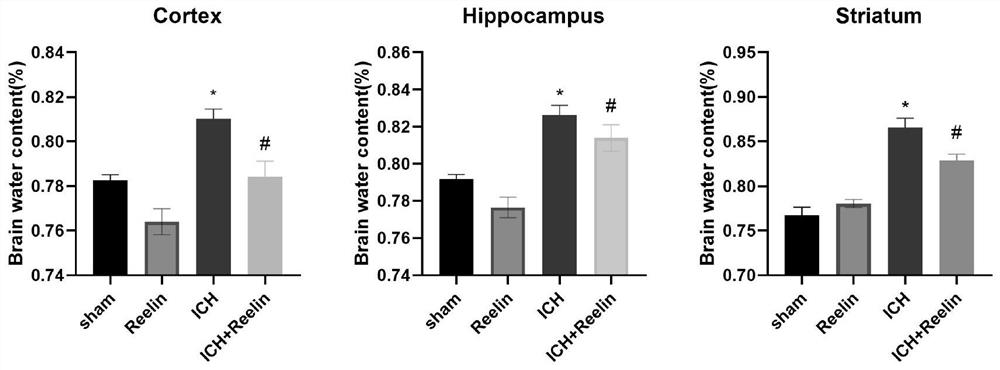
[0023] (1) 24 ICR mice weighing 20-25g were randomly divided into 8 groups (n=3 / group), namely: simple control group (Sham group), 1h, 6h, 12h, 1d, 2d, 3d, 7d group. The modeling steps were as follows: Mice were anesthetized with 4% chloral hydrate by intraperitoneal injection. Depth of anesthesia was observed in mice by eyelid response, skin pinch, or toe reflex. Subsequently, the hair of the mouse head was removed, sterilized with 75% alcohol for several times, the scalp was lifted and cut about 2 cm along the midline, the periosteum was peeled off, and the head of the mouse was fixed with a stereotaxic instrument. Select 1.0mm anterior to the bregma and 2.0mm to the left of the midline, drill the skull with a ball drill to the meninges, pierce the microsyringe vertically 3.5mm, inject 0.5μl of collagenase IV at a constant speed within 5min, and keep the needle for 5min, then withdraw the needle slowly. T...
Embodiment 2
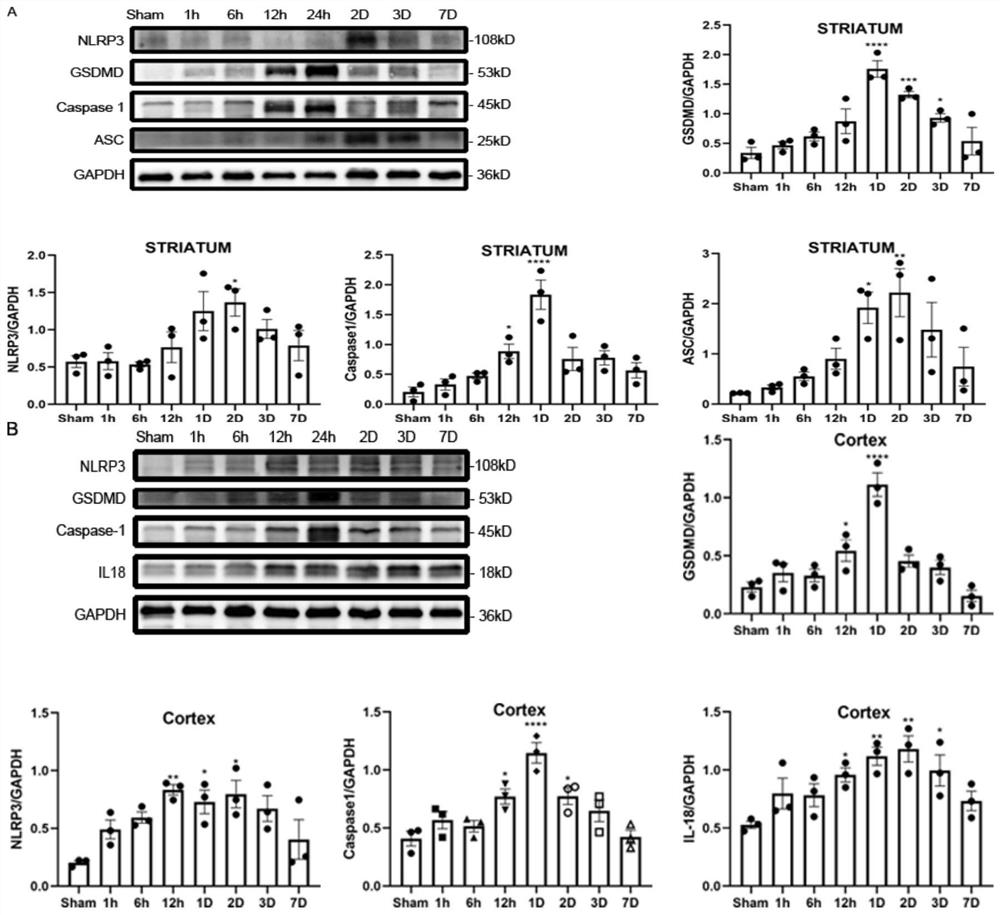
[0026] Example 2: Temporal changes in the expression of pyroptosis-related proteins in different brain regions after intracerebral hemorrhage
[0027] The mice were anesthetized at different time points (1h, 6h, 12h, 1d, 2d, 3d, and 7d) after the intracerebral hemorrhage model was established in the Sham group and 1d after the establishment of the intracerebral hemorrhage model, and the injured side striatum and Cortex, the separated and extracted brain tissue was added to the cell lysate for low temperature homogenization and lysis by ultrasonic cell disruptor, and the supernatant was extracted after low temperature centrifugation for 20 min, and the protein concentration was measured. The proteins were separated by gel electrophoresis. Then, the proteins on the separation gel were transferred to PVDF membranes for 60 min. The PVDF membranes with the proteins transferred were blocked with BSA for 2 h and incubated in BSA-diluted antibodies for 12-14 h at 4°C, followed by PBST....
Embodiment 3
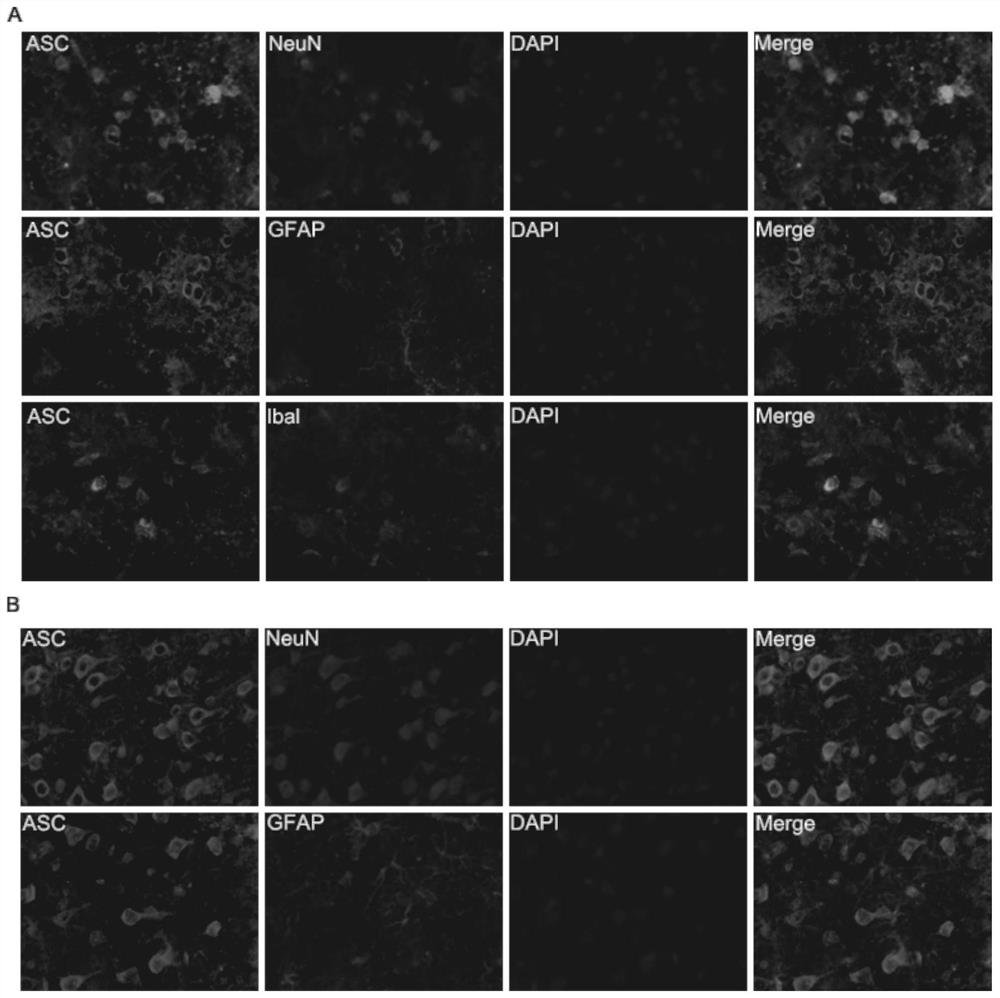
[0029] Example 3: Occurrence and distribution of pyroptosis in different cell types after intracerebral hemorrhage
[0030] Double immunofluorescence staining was performed on the striatum and cortex on the injured side on the 1st day after intracerebral hemorrhage to observe and analyze the relationship between the pyroptosis-related protein ASC and neurons, microglia and astrocytes. The mice were anesthetized 1 day after the establishment of the mouse cerebral hemorrhage model, and the brain tissue was taken out by internal fixation in 4% paraformaldehyde, and then externally fixed for 1 day, followed by sucrose gradient dehydration (20%, 30%, 40%). After the brain tissue sank to the bottom in the sucrose solution, the coronal frozen section of the brain tissue was started. The frozen sections were placed at room temperature for 30 minutes, then fixed in 4% paraformaldehyde for 30 minutes, and washed with PBST solution for several times, five minutes each time. After that, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com