Application of exogenous recombinant protein Reelin in preparing medicament for treating cerebral hemorrhage
A recombinant protein and exogenous technology, applied in the field of biomedicine, can solve problems such as unreported effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
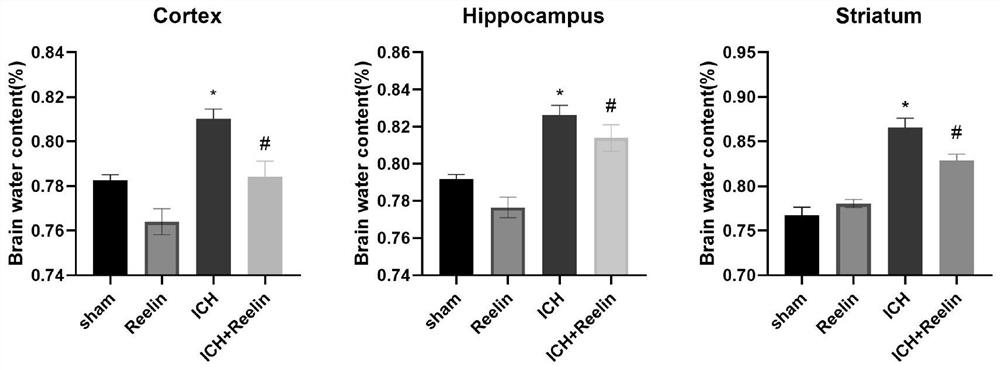
[0022] Example 1: Establishment of cerebral hemorrhage model
[0023] (1) 24 ICR mice weighing 20-25g were randomly divided into 8 groups (n=3 / group), namely: simple control group (Sham group), 1h, 6h, 12h, 1d, 2d, 3d, 7d groups. The modeling steps are as follows: mice were anesthetized by intraperitoneal injection of 4% chloral hydrate. Observe the depth of anesthesia in mice by eyelid response, skin pinch, or toe reflex. Then the hair on the head of the mouse was removed, sterilized several times with 75% alcohol, the scalp was lifted and cut about 2 cm along the midline, the periosteum was peeled off, and the head of the mouse was fixed with a stereotaxic instrument. Select 1.0 mm in front of bregma and 2.0 mm to the left of the midline, drill the skull with a ball drill to the meninges, insert a microinjector vertically 3.5 mm, inject 0.5 μl of collagenase IV at a constant speed within 5 minutes, keep the needle for 5 minutes, and slowly withdraw the needle. The skull o...
Embodiment 2
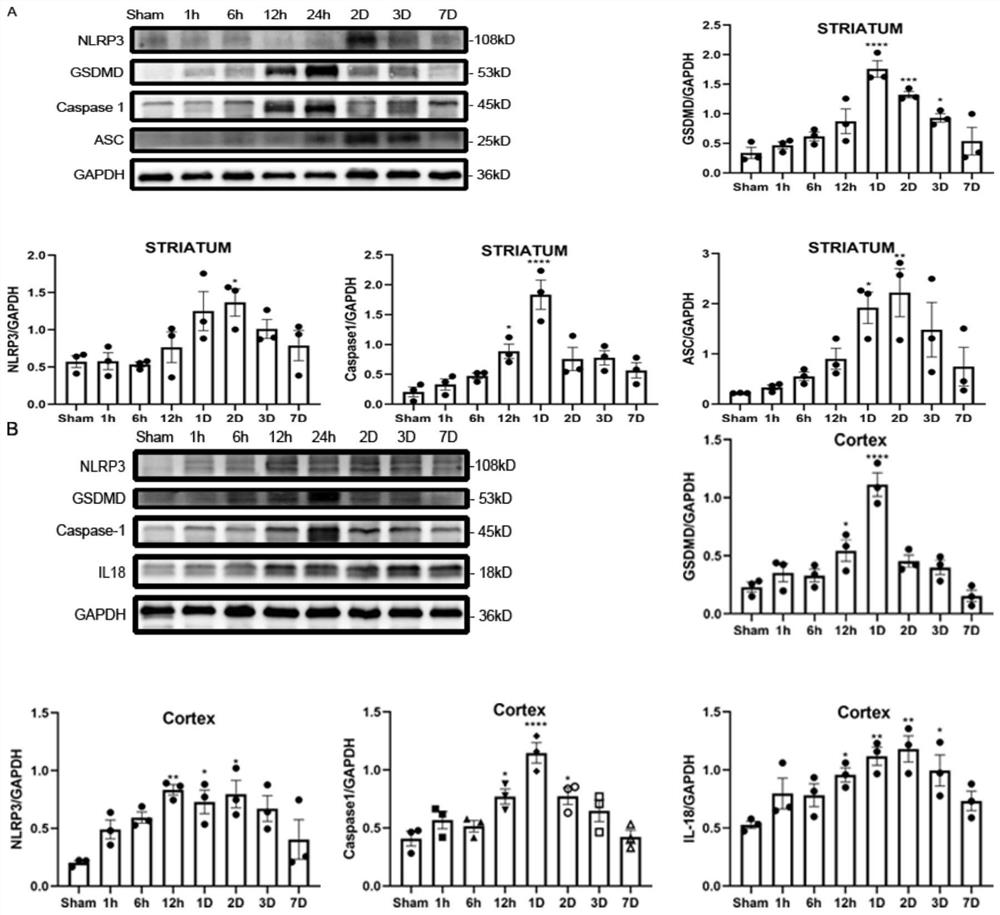
[0026] Example 2: Temporal changes in the expression of pyroptosis-related proteins in different brain regions after cerebral hemorrhage
[0027] According to different time points (1h, 6h, 12h, 1d, 2d, 3d, 7d) after cerebral hemorrhage modeling in mice and 1d after the establishment of cerebral hemorrhage model in Sham group, the mice were anesthetized, and the striatum and striatum on the injured side of the mice were separated respectively. For the cortex, the isolated and extracted brain tissue was added to the cell lysate for low-temperature homogenization and lysis by an ultrasonic cell disruptor, and the supernatant was extracted after low-temperature centrifugation for 20 minutes, and the protein concentration was measured, and an equal amount of 60ug protein samples were loaded in each well using SDS-PAGE Gel electrophoresis to separate the proteins, then transfer the protein on the separation gel to PVDF membrane for 60min, block the PVDF membrane with BSA for 2h and ...
Embodiment 3
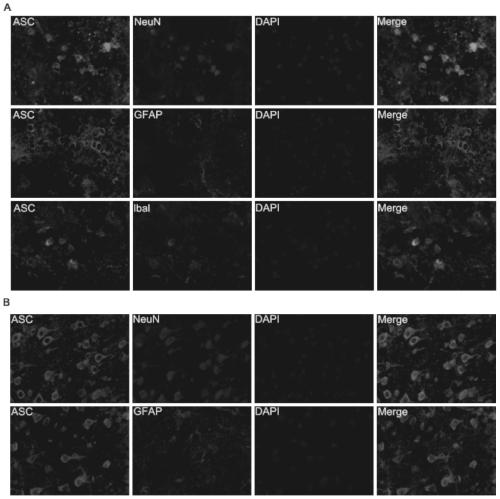
[0029] Example 3: Occurrence and distribution of pyroptosis in different cell types after cerebral hemorrhage
[0030] On the first day after cerebral hemorrhage, double immunofluorescent staining was performed on the striatum and cortex on the injured side, and the relationship between pyroptosis-related protein ASC and neurons, microglia and astrocytes was observed and analyzed. The mice were anesthetized 1 day after the establishment of the mouse intracerebral hemorrhage model, internally fixed with 4% paraformaldehyde, and the brain tissue was taken out, and immediately externally fixed for 1 day, followed by dehydration with sucrose gradient (20%, 30%, 40%). After the brain tissue sank to the bottom in the sucrose solution, the coronal frozen sections of the brain tissue were started, each slice was 10 μm thick, and 50 consecutive sections were sliced at an interval of 200 μm. Frozen sections were placed at room temperature for 30 minutes, then fixed with 4% paraformald...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com